Tumor progression depends on the tumor microenvironment
Researchers from Tokyo Medical and Dental University (TMDU) and Niigata University identify a novel mechanism by which tumors progress
Tokyo, Japan – Tumor cells constantly interact with the cellular environment they live in, affecting tumor progression and metastasis. In a new study, researchers from Tokyo Medical and Dental University (TMDU) and Niigata University discovered that the proteins transforming growth factor-β (TGF-β) and tumor necrosis factor alpha (TNF-α) promote the development of cancer-associated fibroblasts, which in turn contribute to tumor progression.
Tumor cells live within a specific tumor microenvironment, in which they are surrounded by other cell types, including those comprising blood vessels, cancer-associated fibroblasts (CAFs) and inflammatory cells. The tumor microenvironment consists of a network of molecules that provide structural and biochemical support to all cells. One such molecule is TGF-β, which is known to induce endothelial-mesenchymal transition (EndMT), a process that involves the conversion of endothelial cells, which comprise the inner lining of blood vessels, to CAFs, which modulate the tumor progression.
“We have previously shown that CAFs derived from EndMT promote tumor formation and progression. However, we do not know much about the molecular mechanisms underlying these interactions,” says corresponding author of the study Tetsuro Watabe (TMDU). “The goal of our study was to investigate how the tumor microenvironment contributes to tumor progression.”
To achieve their goal, the researchers focused on the protein TNF-α, a known cytokine secreted by inflammatory cells. Because tumors are often infiltrated by inflammatory cells, the tumor microenvironment contains high levels of TNF-α. To understand the roles of TNF-α in TGF-β-induced EndMT, the researchers treated human endothelial cells with TGF-β, TNF-α, or both. TGF-β robustly induced EndMT as shown through increased expression of various markers of CAFs as well as a transition towards a CAF morphology of the human endothelial cells. However, interestingly enough, TNF-α enhanced the molecular effects of TGF-β.
Because one of the main biological functions of CAFs is to secrete proteins into the tumor microenvironment and induce tumor progression, the researchers next cultured human oral cancer cells in the presence of the proteins secreted from EndMT-derived CAFs. The researchers found that oral cancer cells underwent epithelial-mesenchymal transition (EMT). Since EMT is a hallmark of tumor progression and metastasis, these results demonstrated that the proteins secreted by CAFs contribute to tumor progression. The researchers also found that these effects of CAFs on tumor progression was suppressed by inhibition of TGF-β, suggesting that TGF-β protein secreted from CAFs induced EMT.
“These are striking results that identify a molecular mechanism underlying the role of the tumor microenvironment in tumor biology. We hope that our findings will aid in the development of novel cancer therapies,” says first and co-corresponding author Yasuhiro Yoshimatsu (Niigata University).
Tokyo, Japan – Tumor cells constantly interact with the cellular environment they live in, affecting tumor progression and metastasis. In a new study, researchers from Tokyo Medical and Dental University (TMDU) and Niigata University discovered that the proteins transforming growth factor-β (TGF-β) and tumor necrosis factor alpha (TNF-α) promote the development of cancer-associated fibroblasts, which in turn contribute to tumor progression.
Tumor cells live within a specific tumor microenvironment, in which they are surrounded by other cell types, including those comprising blood vessels, cancer-associated fibroblasts (CAFs) and inflammatory cells. The tumor microenvironment consists of a network of molecules that provide structural and biochemical support to all cells. One such molecule is TGF-β, which is known to induce endothelial-mesenchymal transition (EndMT), a process that involves the conversion of endothelial cells, which comprise the inner lining of blood vessels, to CAFs, which modulate the tumor progression.
“We have previously shown that CAFs derived from EndMT promote tumor formation and progression. However, we do not know much about the molecular mechanisms underlying these interactions,” says corresponding author of the study Tetsuro Watabe (TMDU). “The goal of our study was to investigate how the tumor microenvironment contributes to tumor progression.”
To achieve their goal, the researchers focused on the protein TNF-α, a known cytokine secreted by inflammatory cells. Because tumors are often infiltrated by inflammatory cells, the tumor microenvironment contains high levels of TNF-α. To understand the roles of TNF-α in TGF-β-induced EndMT, the researchers treated human endothelial cells with TGF-β, TNF-α, or both. TGF-β robustly induced EndMT as shown through increased expression of various markers of CAFs as well as a transition towards a CAF morphology of the human endothelial cells. However, interestingly enough, TNF-α enhanced the molecular effects of TGF-β.
Because one of the main biological functions of CAFs is to secrete proteins into the tumor microenvironment and induce tumor progression, the researchers next cultured human oral cancer cells in the presence of the proteins secreted from EndMT-derived CAFs. The researchers found that oral cancer cells underwent epithelial-mesenchymal transition (EMT). Since EMT is a hallmark of tumor progression and metastasis, these results demonstrated that the proteins secreted by CAFs contribute to tumor progression. The researchers also found that these effects of CAFs on tumor progression was suppressed by inhibition of TGF-β, suggesting that TGF-β protein secreted from CAFs induced EMT.
“These are striking results that identify a molecular mechanism underlying the role of the tumor microenvironment in tumor biology. We hope that our findings will aid in the development of novel cancer therapies,” says first and co-corresponding author Yasuhiro Yoshimatsu (Niigata University).
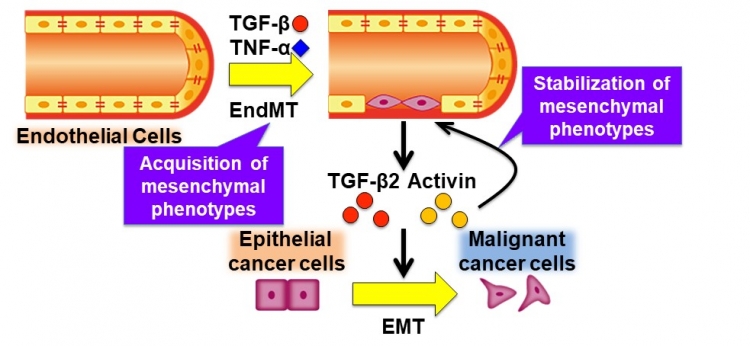
Roles of tumor microenvironment networks during tumor progression
The tumor microenvironment (TME) consists of various components, including cancer cells, tumor vessels, cancer-associated fibroblasts (CAFs), and inflammatory cells. These components interact with each other via various cytokines including TGF-β and TNF-α, which often induce tumor progression. In the present study, we showed that TGF-β and TNF-α induce the endothelial-to-mesenchymal transition (EndMT), in which endothelial cells (ECs) acquire mesenchymal phenotypes. The ECs that have undergone EndMT, in turn, secrete TGF-β2 and Activin by themselves. The irreversible and long-lasting effects by the cytokines result in the stabilization of mesenchymal phenotypes of ECs. The secreted cytokines derived from the ECs also induce the epithelial-to-mesenchymal transition (EMT) of epithelial cancer cells, which contributes to tumor progression.
The tumor microenvironment (TME) consists of various components, including cancer cells, tumor vessels, cancer-associated fibroblasts (CAFs), and inflammatory cells. These components interact with each other via various cytokines including TGF-β and TNF-α, which often induce tumor progression. In the present study, we showed that TGF-β and TNF-α induce the endothelial-to-mesenchymal transition (EndMT), in which endothelial cells (ECs) acquire mesenchymal phenotypes. The ECs that have undergone EndMT, in turn, secrete TGF-β2 and Activin by themselves. The irreversible and long-lasting effects by the cytokines result in the stabilization of mesenchymal phenotypes of ECs. The secreted cytokines derived from the ECs also induce the epithelial-to-mesenchymal transition (EMT) of epithelial cancer cells, which contributes to tumor progression.
###
The article, “TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation,” was published in Cancer Science at DOI: 10.1111/cas.14455
The article, “TNF-α enhances TGF-β-induced endothelial-to-mesenchymal transition via TGF-β signal augmentation,” was published in Cancer Science at DOI: 10.1111/cas.14455
Summary
Researchers from Tokyo Medical and Dental University (TMDU) and Niigata University identified a novel mechanism by which tumors progress. By studying the role of TNF-α and TGF-β in the formation of cancer-associated fibroblasts (CAFs), the researchers found that both proteins together exert a robust effect on the development of CAFs. They further found that oral cancer cells show increased tumor progression in response to TGF-β protein secreted from CAFs. The findings of this study could help develop novel cancer therapies.
Correspondence to
Tetsuro WATABE, Professor
Department of Biochemistry,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:t-watabe.bch(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.
Department of Biochemistry,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:t-watabe.bch(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

