A Matter of Concentration: Molecular Mechanisms of Water Homeostasis
July 22, 2022
Researchers led by Tokyo Medical and Dental University (TMDU) elucidate a key protein interaction involved in the process of urine concentration
Tokyo, Japan – When making a soup, maintaining the correct balance of liquid and salt is key. Similarly, maintaining the balance of water and electrolytes is an important physiological process in the human body, and disruption of this process can have serious consequences. The kidneys regulate the amount of water and solutes taken in and excreted from the body through a process known as urine concentration. Recently, researchers in Japan have shed new light on the molecular mechanisms underlying this process.
In a new study published in PNAS, researchers led by Tokyo Medical and Dental University (TMDU) have identified the role of a key protein, lipopolysaccharide-responsive and beige-like anchor protein (LRBA), in the process of urine concentration. LRBA was found to regulate the function of protein kinase A (PKA), a signaling molecule involved in a diverse range of biological processes.
In the kidneys, PKA is known to regulate aquaporin-2 (AQP2) water channels that are activated in response to the antidiuretic hormone vasopressin during dehydration and facilitate water reabsorption, thereby preventing further water loss from the body. However, the specifics of the interaction between PKA and AQP2 remained unclear. To further understand the molecular mechanisms involved in this regulation process, the TMDU-led research team investigated potential mediators of PKA in the kidneys in the hope to find a missing step in AQP2 regulation.
“We performed a screen of PKA substrates in mouse kidney cells to determine proteins that correlate with AQP2,” says lead author Fumiaki Ando. “We identified LRBA as a candidate that corresponded with AQP2 activation.”
Further analysis showed that LRBA co-localized with AQP2 in the renal collecting ducts – a section of the kidney that is involved in water reclamation. The research team evaluated mice lacking LRBA and found that they exhibited polyuria (excessive urination) and impaired activation of AQP2.
“Our results indicated that LRBA interacts with PKA to anchor it in the renal collecting ducts and thereby facilitates PKA-induced activation of AQP2,” says senior author Shinichi Uchida. “In the absence of LRBA, AQP2 is not activated, resulting in a decrease in water reabsorption and an increase in urine output.”
The research team’s findings demonstrate that LRBA is essential for the maintenance of water homeostasis in the body, providing new insight into the molecular mechanisms of urine concentration (Figure). Notably, the LRBA–PKA interaction may serve as a potential target for the development of drug treatments for polyuria.
In a new study published in PNAS, researchers led by Tokyo Medical and Dental University (TMDU) have identified the role of a key protein, lipopolysaccharide-responsive and beige-like anchor protein (LRBA), in the process of urine concentration. LRBA was found to regulate the function of protein kinase A (PKA), a signaling molecule involved in a diverse range of biological processes.
In the kidneys, PKA is known to regulate aquaporin-2 (AQP2) water channels that are activated in response to the antidiuretic hormone vasopressin during dehydration and facilitate water reabsorption, thereby preventing further water loss from the body. However, the specifics of the interaction between PKA and AQP2 remained unclear. To further understand the molecular mechanisms involved in this regulation process, the TMDU-led research team investigated potential mediators of PKA in the kidneys in the hope to find a missing step in AQP2 regulation.
“We performed a screen of PKA substrates in mouse kidney cells to determine proteins that correlate with AQP2,” says lead author Fumiaki Ando. “We identified LRBA as a candidate that corresponded with AQP2 activation.”
Further analysis showed that LRBA co-localized with AQP2 in the renal collecting ducts – a section of the kidney that is involved in water reclamation. The research team evaluated mice lacking LRBA and found that they exhibited polyuria (excessive urination) and impaired activation of AQP2.
“Our results indicated that LRBA interacts with PKA to anchor it in the renal collecting ducts and thereby facilitates PKA-induced activation of AQP2,” says senior author Shinichi Uchida. “In the absence of LRBA, AQP2 is not activated, resulting in a decrease in water reabsorption and an increase in urine output.”
The research team’s findings demonstrate that LRBA is essential for the maintenance of water homeostasis in the body, providing new insight into the molecular mechanisms of urine concentration (Figure). Notably, the LRBA–PKA interaction may serve as a potential target for the development of drug treatments for polyuria.
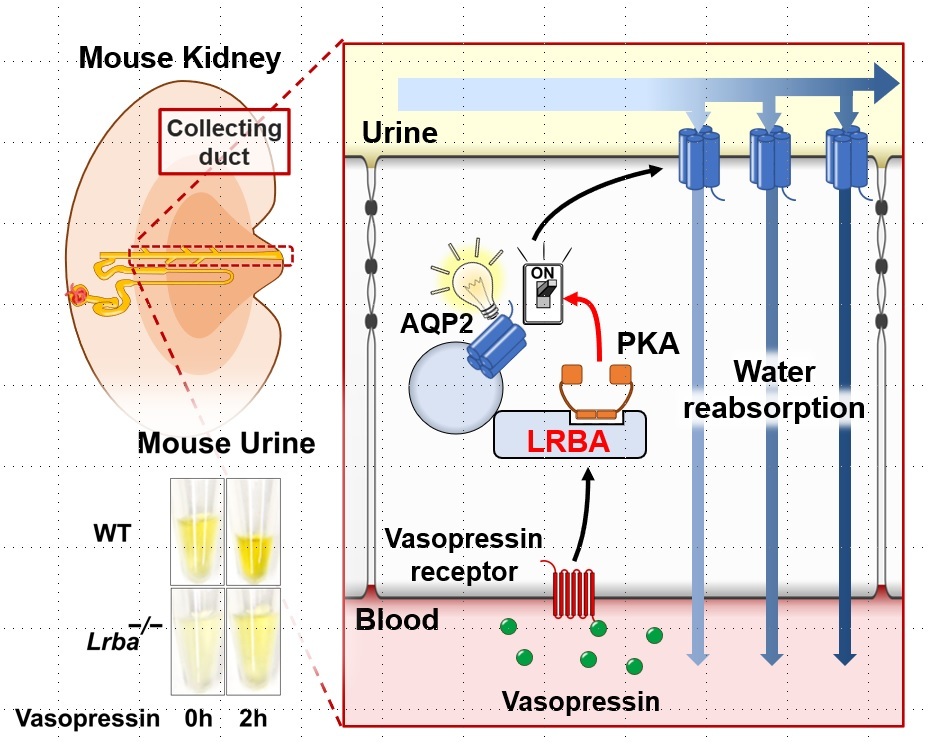
LRBA assists PKA to switch on AQP2 water channels for the prevention of further water loss from the body during dehydration.
###
The article, “LRBA is essential for urinary concentration and body water homeostasis,” was published in PNAS at DOI: 10.1073/pnas.2202125119.
Summary
Researchers led by Tokyo Medical and Dental University (TMDU) identified the role of lipopolysaccharide-responsive and beige-like anchor protein (LRBA) in the process of urine concentration in the kidneys. Through interaction with protein kinase A, LRBA regulates the activation of aquaporin-2 water channels, which facilitate the absorption of water during dehydration. The LRBA–protein kinase A interaction may serve as a potential target for the development of anti-aquaretic drugs.
Journal Article
JOURNAL:Proceedings of the National Academy of Sciences of the United States of America
TITLE:LRBA is essential for urinary concentration and body water homeostasis
DOI:https://doi.org/10.1073/pnas.2202125119
TITLE:LRBA is essential for urinary concentration and body water homeostasis
DOI:https://doi.org/10.1073/pnas.2202125119
Correspondence to
Shinichi Uchida,PhD,Professor
Fumiaki Ando,Assistant Professor
Department of Nephrology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:fandkidc(at)tmd.ac.jp
*Please change (at) in the e-mail addresses to @ when sending correspondence.

