Cannabidiol suppresses the induction of neoplastic osteoclasts – Aiming to develop new therapeutic agents against cancer-induced bone destruction—
Key Points
| ● While osteoclasts are differentiated from macrophages by osteoclast-inducing factor RANKL, it was found that osteoclast differentiation can occur from osteoclast precursor cells via a different mechanism. |
| ● Using the osteoclast-induction system of oral squamous cell carcinoma, cannabidiol, a type of cannabinoid, was shown to almost completely inhibit osteoclast induction. |
| ● According to the results of this study, osteoclast precursor cells may be a new therapeutic target for the treatment of cancer-induced bone destruction. |
A research group led by Professor Tohru Ikeda and graduate student, Maiko Tsuchiya of the Department of Oral Pathology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University demonstrated that while almost no osteoclast was induced after co-culture of human oral squamous cell carcinoma with mouse-derived macrophages, a large number of osteoclasts were induced from osteoclast-precursor cells destined to become osteoclasts by weak stimulation of osteoclast-inducing factor RANKL, with extracellular vesicles secreted by cancer cells being responsible for this osteoclast induction. The research group also demonstrated that this type of induction of osteoclast from osteoclast-precursor cells is due to a newly discovered mechanism that does not rely on the increased expression of NFATc1, a master transcription factor*1 in osteoclast induction. Furthermore, although the widely used bone resorption inhibitor denosumab (human RANKL neutralizing antibody) was ineffective in inhibiting this type of osteoclast induction, a drug known as cannabidiol almost completely inhibited this induction. The present study demonstrated for the first time ever that osteoclast differentiation from osteoclast-precursor cells may occur via a variety of mechanisms and may provide clues to the development of new treatments for cancer-induced bone destruction. The findings were accepted by the International Journal of Molecular Sciences on December 6, 2019 and were published online on December 9, 2019.
Background of Research
Osteoclasts are cells that resorb bone, maintaining bone metabolic balance and bone health in collaboration with osteoblasts, which form bone. When the balance inclines towards resorption dominance, excessive bone resorption occurs, leading to osteoporosis. Tipping the balance towards bone formation may also lead to diseases involving abnormal bone hardening. It is known that when the macrophage, derived from white blood cell known as a monocyte outside the vessel, is stimulated by the osteoclast-inducing factor RANKL, osteoclasts are induced through signal transmission to the nucleus, which in turn enhances the expression of the master transcription factor NFATc1. For this reason, denosumab is widely used as a treatment for excessive bone resorption due to bone metastasis or osteoporosis.
However, treatments inhibiting RANKL produce side-effects, and suppress systemic bone metabolism to increase the risk of intractable osteomyelitis of the jaw, severely limiting dental treatments, such as tooth extraction, in patients receiving this medication. Moreover, the number of patients experiencing this side-effect, known as medication-related osteonecrosis of the jaw (MRONJ), is rising along with the increase in elderly patients. MRONJ is especially likely to occur in patients receiving treatment for bone metastases, thus prompting a strong demand for the development of a drug capable of specifically suppressing cancer-induced excessive bone resorption without inhibiting systemic bone metabolism.
However, treatments inhibiting RANKL produce side-effects, and suppress systemic bone metabolism to increase the risk of intractable osteomyelitis of the jaw, severely limiting dental treatments, such as tooth extraction, in patients receiving this medication. Moreover, the number of patients experiencing this side-effect, known as medication-related osteonecrosis of the jaw (MRONJ), is rising along with the increase in elderly patients. MRONJ is especially likely to occur in patients receiving treatment for bone metastases, thus prompting a strong demand for the development of a drug capable of specifically suppressing cancer-induced excessive bone resorption without inhibiting systemic bone metabolism.
Outline of Research
Based on findings from co-culture of human oral squamous cell carcinoma cells with murine macrophages and from application of the culture supernatants of the cancer cells to murine macrophages, the research group reported that a hitherto unknown mechanism may contribute to cancer cell-mediated osteoclast induction from osteoclast precursor cells destined to become osteoclasts via weak stimulation by RANKL, not from macrophages which are capable of differentiating into a variety of cell types besides osteoclasts and that RANKL inhibition is not effective for preventing osteoclast induction from osteoclast precursor cells, mediated by oral squamous cell carcinoma cells (Wada et al., Biochem. Biophys. Res. Commun. 509: 108-113, 2019). In the present study, extracellular vesicles secreted by oral squamous cell carcinoma cell 3A, which can induce osteoclasts from osteoclast precursor cells, and oral squamous cell carcinoma cell HO-1-N1, which cannot induce osteoclasts were collected. The results showed that the extracellular vesicles of 3A cells possessed the ability to induce osteoclasts while those of HO-1-N1 did not (Figure 1).
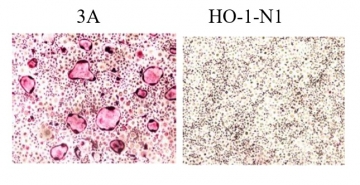
Figure 1: When 10 micrograms/mL of extracellular vesicles collected from oral squamous cell carcinoma cells 3A and H0-1-N1 were added to osteoclast precursor cells, the osteoclast-inducing potential was demonstrated only for extracellular vesicles derived from oral squamous cell carcinoma cell 3A.
The inefficacy of denosumab was confirmed against this osteoclast induction mediated by the extracellular vesicles derived from this type of cancer cells. Several drugs known to inhibit extracellular vesicle secretion were examined for their effect on osteoclast induction for the purpose of inhibiting osteoclast induction mediated by the extracellular vesicles. The results showed one of the drugs, cannabidiol, was able to almost completely inhibit osteoclast induction against which denosumab was ineffective (Figure 2).
The inefficacy of denosumab was confirmed against this osteoclast induction mediated by the extracellular vesicles derived from this type of cancer cells. Several drugs known to inhibit extracellular vesicle secretion were examined for their effect on osteoclast induction for the purpose of inhibiting osteoclast induction mediated by the extracellular vesicles. The results showed one of the drugs, cannabidiol, was able to almost completely inhibit osteoclast induction against which denosumab was ineffective (Figure 2).
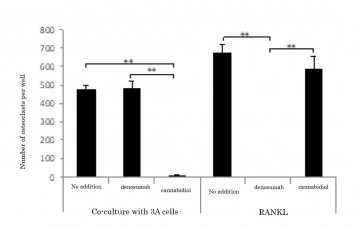
Figure 2: Inhibitory effects on osteoclast induction by co-culture of osteoclast precursor cells with 3A cells and RANKL addition.
Co-culture of osteoclast precursor cells with 3A cells induced the formation of a large number of osteoclasts. Denosumab was ineffective against this osteoclast induction, but cannabidiol showed an almost complete inhibitory effect. On the other hand, Denosumab was completely effective in inhibiting osteoclasts induced by the addition of RANKL whereas cannabidiol was not.
Cannabidiol is used abroad clinically mainly as a therapeutic agent for neurological diseases. Moreover, physiologically produced endocannabinoids are attracting attention for having an important role in regulating bone metabolism. Contrary to expectations, the addition of cannabidiol failed completely to inhibit extracellular vesicle secretion from oral squamous cell carcinoma cells. In addition, removal of extracellular vesicles from the culture supernatants of 3A cells could not completely eliminate the osteoclast-inducing ability, suggesting that not only the extracellular vesicles, but also other cancer cell-derived factors may be at work in oral squamous cell carcinoma cell-mediated osteoclast induction from osteoclast precursor cells. Nonetheless, cannabidiol was found to be able to inhibit the induction of osteoclasts mediated by this type of cancer cells almost completely.
Although the details of the mechanism of osteoclast induction inhibited by cannabidiol are still unclear, the present study also revealed that this mechanism is unrelated to NFATc1 as the master transcription factor for the osteoclast induction, whose expression is enhanced by RANKL stimulation. This suggested a yet unidentified mechanism.
Until now, the increased expression of NFATc1 via the activity of RANKL in macrophages was thought to be the sole route of osteoclast induction. However, the present study demonstrated that RANKL is essential only for initial phase, where osteoclast precursor cells destined to become osteoclasts are induced from macrophages, and that another yet unidentified pathway beginning with osteoclast precursor cells may be involved in osteoclast induction.
Co-culture of osteoclast precursor cells with 3A cells induced the formation of a large number of osteoclasts. Denosumab was ineffective against this osteoclast induction, but cannabidiol showed an almost complete inhibitory effect. On the other hand, Denosumab was completely effective in inhibiting osteoclasts induced by the addition of RANKL whereas cannabidiol was not.
Cannabidiol is used abroad clinically mainly as a therapeutic agent for neurological diseases. Moreover, physiologically produced endocannabinoids are attracting attention for having an important role in regulating bone metabolism. Contrary to expectations, the addition of cannabidiol failed completely to inhibit extracellular vesicle secretion from oral squamous cell carcinoma cells. In addition, removal of extracellular vesicles from the culture supernatants of 3A cells could not completely eliminate the osteoclast-inducing ability, suggesting that not only the extracellular vesicles, but also other cancer cell-derived factors may be at work in oral squamous cell carcinoma cell-mediated osteoclast induction from osteoclast precursor cells. Nonetheless, cannabidiol was found to be able to inhibit the induction of osteoclasts mediated by this type of cancer cells almost completely.
Although the details of the mechanism of osteoclast induction inhibited by cannabidiol are still unclear, the present study also revealed that this mechanism is unrelated to NFATc1 as the master transcription factor for the osteoclast induction, whose expression is enhanced by RANKL stimulation. This suggested a yet unidentified mechanism.
Until now, the increased expression of NFATc1 via the activity of RANKL in macrophages was thought to be the sole route of osteoclast induction. However, the present study demonstrated that RANKL is essential only for initial phase, where osteoclast precursor cells destined to become osteoclasts are induced from macrophages, and that another yet unidentified pathway beginning with osteoclast precursor cells may be involved in osteoclast induction.
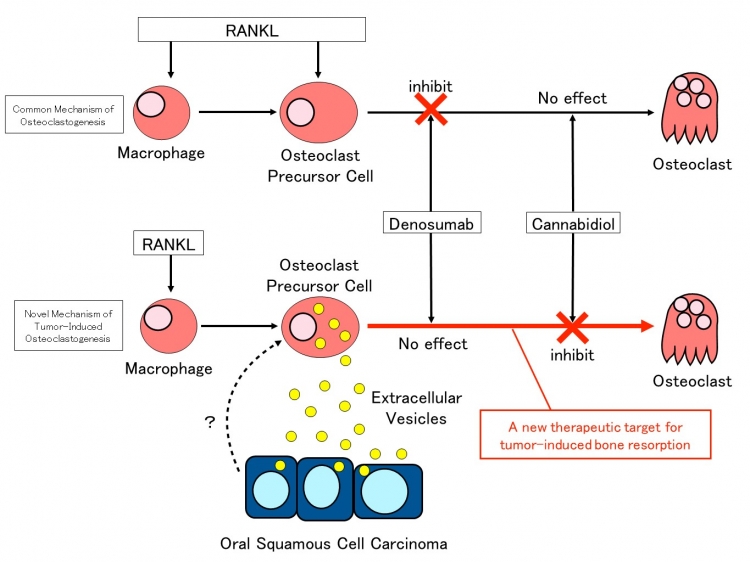
Figure 3: Summary of the study
Extracellular vesicles released by oral squamous cell carcinoma cells induce osteoclast differentiation from osteoclast precursor cells. While denosumab was ineffective against osteoclast differentiation in this mechanism, cannabidiol demonstrated an inhibitory effect.
Extracellular vesicles released by oral squamous cell carcinoma cells induce osteoclast differentiation from osteoclast precursor cells. While denosumab was ineffective against osteoclast differentiation in this mechanism, cannabidiol demonstrated an inhibitory effect.
Significance of Research Findings
This study produced three major findings. First, it was demonstrated for the first time ever that osteoclast induction from osteoclast precursor cells may be governed by a previously unknown mechanism. While previous studies on genetically modified mice have already shown that RANKL is essential for osteoclast induction, the present study demonstrated that RANKL is essential only in the early stage of osteoclast induction at which macrophages are destined to be differentiated into osteoclasts. Second, the study demonstrated that extracellular vesicles secreted by cancer cells induced osteoclasts. Extracellular vesicles play a role in intercellular communication, and while much research has been done with cancer cells in this respect, the present study showed for the first time that the extracellular vesicles of oral squamous cell carcinoma cells induced osteoclasts from osteoclast precursor cells. Third, the study demonstrated that cannabidiol almost completely inhibited osteoclast induction mediated by this type of cancer cells. It is hoped that these findings will spur the development of innovative treatments specifically inhibiting cancer-induced bone destruction.
Glossary
*1 Master transcription factor
A molecule that directly induces the expression of genes essential for cell differentiation and function.
A molecule that directly induces the expression of genes essential for cell differentiation and function.
Article Information
Journal : International Journal of Molecular Sciences
Article title : A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol
DOI:
https://doi.org/10.3390/ijms20246211
Article title : A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol
DOI:
https://doi.org/10.3390/ijms20246211
Researcher Profile

Tohru Ikeda, Professor
Department of Oral Pathology
Tokyo Medical and Dental University
Research area: Experimental pathology, clinical pathological diagnosis
Department of Oral Pathology
Tokyo Medical and Dental University
Research area: Experimental pathology, clinical pathological diagnosis
Contact Information
【Regarding research】
Tohru Ikeda
Department of Oral Pathology,
Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University
TEL: +81-3-5803-5451
FAX: +81-3-5803-0188
E-mail: tohrupth.mpa@tmd.ac.jp
【Regarding press】
Public Relations Division
Tokyo Medical and Dental University
1-5-45 Yushima
Bunkyou-ku, Tokyo 113-8510
TEL: +81-3-5803-5833
FAX: +81-3-5803-0272
E-mail: kouhou.adm@tmd.ac.jp
Tohru Ikeda
Department of Oral Pathology,
Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University
TEL: +81-3-5803-5451
FAX: +81-3-5803-0188
E-mail: tohrupth.mpa@tmd.ac.jp
【Regarding press】
Public Relations Division
Tokyo Medical and Dental University
1-5-45 Yushima
Bunkyou-ku, Tokyo 113-8510
TEL: +81-3-5803-5833
FAX: +81-3-5803-0272
E-mail: kouhou.adm@tmd.ac.jp

