Sweet move: a modified sugar enhances antisense oligonucleotide safety and efficacy
Tokyo, Japan – Diseases that affect the brain and spinal cord can be particularly devastating, and finding new and more effective ways to treat these conditions is an important goal for researchers and clinicians alike. Now, a research group from Japan reports that slightly modifying an existing treatment for central nervous system (CNS) disease dramatically increases its effectiveness.
In a study published recently in Molecular Therapy Nucleic Acids, researchers from Tokyo Medical and Dental University (TMDU) and Osaka University have revealed that adding a modified sugar to antisense oligonucleotides (ASOs), a recently developed treatment strategy for CNS disease caused by toxic proteins, greatly decreases disease symptoms.
Multiple ASOs have been approved for clinical use, including gapmer ASOs, which are small pieces of genetic material binding to RNA messenger molecules that produce mutant disease-causing proteins to flag them for degradation. Modifying the chemical composition of these gapmer ASOs can both increase their ability to target molecules for degradation and decrease the toxic side effects of the treatment.
“We recently developed a new chemical modification called BNAP-AEO,” says lead author of the study Taiki Matsubayashi. “While ASOs carrying BNAP-AEO are expected to be highly effective, their biological efficacy and toxicity have not been investigated.”
To characterize gapmer ASOs modified by the inclusion of BNAP-AEO, the researchers first evaluated the stability of gapmer ASO binding to target molecules at different temperatures.
They then tested the ability of these modified gapmer ASOs to block the production of disease-causing proteins in brain cancer cells and in mice.
“The results were very exciting,” explains Takanori Yokota, senior author. “Not only did ASOs carrying BNAP-AEO have a higher binding affinity for the target than those without BNAP-AEO, they also induced more efficient gene silencing in vitro and strongly suppressed gene expression in the mouse brain.”
Furthermore, modifying gapmer ASOs with BNAP-AEO decreased their toxic side effects in mice, possibly by changing their interactions with receptors on the surface of cells in the brain and spinal cord.
“Our findings highlight the efficient gene-silencing effect of ASOs incorporating BNAP-AEO, as well as an unexpected role for this modification in decreasing CNS toxicity,” says Matsubayashi.
In addition to providing a new way to increase the efficacy and safety of gapmer ASOs, the results from this study suggest that ASOs that bind stably at high temperatures are not necessarily more potent, as is commonly thought. This could be explained by the BNAP-AEO chemical modification affecting other features of the ASOs, such as which cell compartment they localize to.
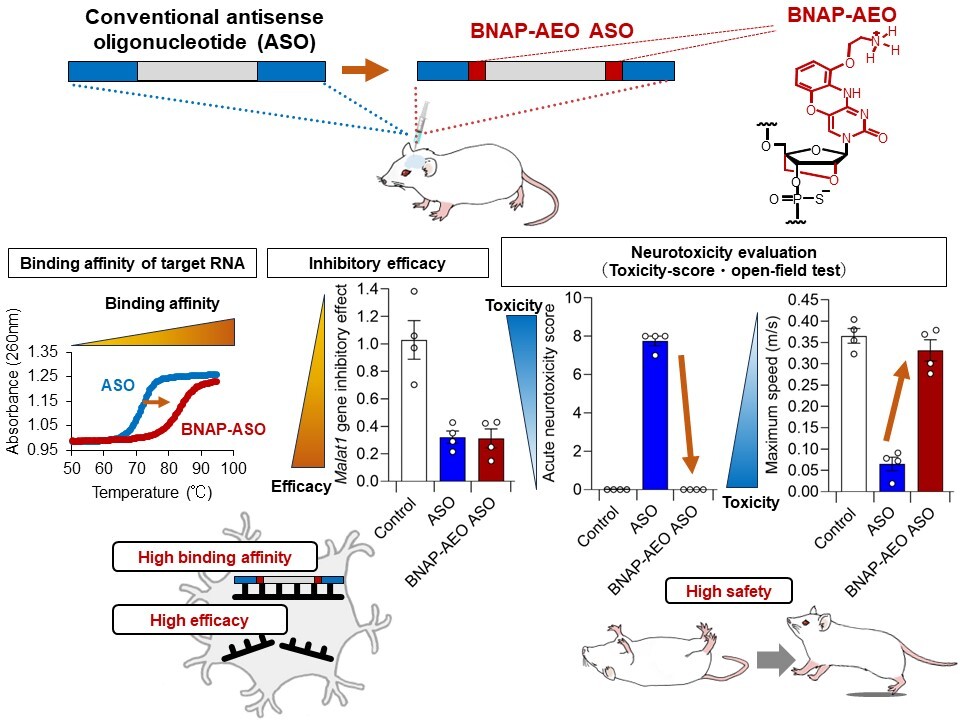
Figure A:The BNAP-AEO ASO enables both high efficacy and safety.
We designed and synthesized both a conventional ASO and a ASO modified with BNAP-AEO (BNAP-AEO ASO) (top row). The ASO with BNAP-AEO exhibited a higher binding affinity to the target RNA than the conventional ASO (middle left). Additionally, the BNAP-AEO administered into mice intrathecally showed lower neurotoxicity compared to the conventional ASO without BNAP-AEO with evaluation by toxicity score as well as assessment of spontaneous motor function (open field test), while maintaining the inhibitory effect on the target gene (middle right). In other words, BNAP-AEO demonstrates high binding affinity, efficacy, and safety simultaneously.
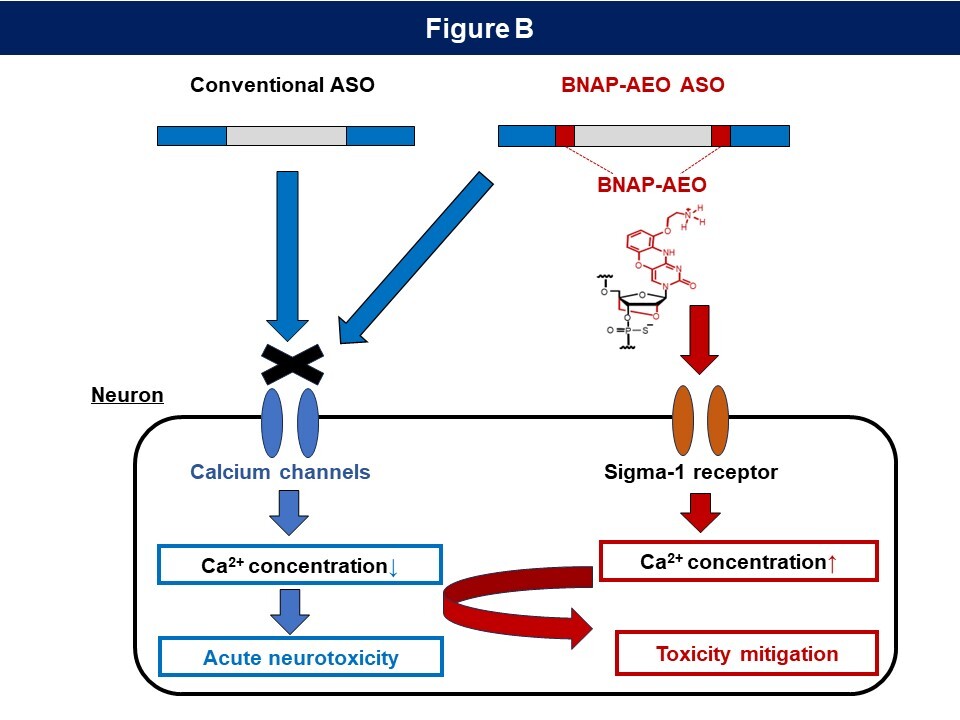
Figure B:Hypothesis on the molecular mechanism underlying the mitigation of neurotoxicity by BNAP-AEO.
The conventional ASO inhibits the function of calcium channels in neurons, inducing a decrease in intracellular calcium ions and the manifestation of acute neurotoxicity (left). In contrast, the BNAP-AEO ASO prevents the reduction of intracellular calcium ion via the Sigma-1 receptor, potentially improving the induced toxicity (right).
Summary
Journal Article
TITLE:Favorable Efficacy and Reduced Acute Neurotoxicity by Antisense Oligonucleotides with 2',4'-BNA/LNA with 9-(aminoethoxy)phenoxazine
DOI:https://doi.org/10.1016/j.omtn.2024.102161
Correspondence to
Takanori, Yokota, Professor
Department of Neurology and Neurological Science,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:tak-yokota.nuro(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

