“Identification of the commander cell in the bone remodeling”

Hiroshi Takayanagi
Professor, Department of Cell Signaling,
Graduate School of Medical and Dental Sciences (right)
Tomoki Nakashima
Assistant Professor,
Department of Cell Signaling,
Graduate School of Medical and Dental Sciences (left)
Point
| ● | Bone is constantly renewed by the balanced action of osteoblastic bone formation and osteoclastic bone resorption at the bone surface. This process called "bone remodeling" is important not only for maintaining bone mass and strength, but also for mineral homeostasis. Here we show that osteocytes embedded within the bone matrix are the commander cell at the initiation of the bone remodeling through regulation of osteoclastogenesis. |
| ● | Using a newly established method for the isolation of high purity osteocytes from bone, we show that osteocytes express a much higher amount of RANKL and have a much greater capacity to support osteoclastogenesis than osteoblasts and bone marrow stromal cells. The crucial role of RANKL expressed by osteocytes was validated by the severe osteopetrotic phenotype observed in mice lacking RANKL specifically in osteocytes. |
Background
Content of the research
Future potential
Contact
Hiroshi Takayanagi, M.D., Ph.D.
Department of Cell Signaling, Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University
1-5-45 Yushima, Bunkyo-ku, Tokyo, Japan 113-8510
Phone: +81-3-5803-5471
Fax: +81-3-5803-0192
E-mail: taka.csi(at)tmd.ac.jp
Takayanagi Lab HP: http://osteoimmunology.com/
[About action of Japan Science and Technology Agency]
Department of Research Project, Innovation Headquarters, Japan Science and Technology Agency
Hiroyuki Kaneko
Sanban-cho bldg., 5, Sanban-cho, Chiyoda-ku, Tokyo, Japan 102-0075
Phone: +81-3-5803-5471
Fax: +81-3-5803-0192
E-mail: hkaneko(at)jst.go.jp
ERATO Takayanagi Osteonetwork Project HP: http://www.osteonetwork.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.
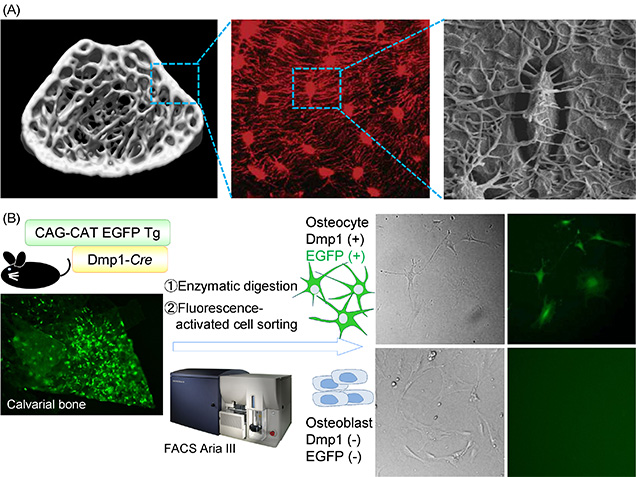
Figure 1:Development of an isolation method to provide osteocyte of high purity.
(A) Osteocyte is the most abundant cell type of bone. The cells embedded within the bone matrix connect with each other and with osteoblasts or/and osteoclasts at the bone surface through a network of canaliculi (Image of fluorescence [Nat Genet 2006] and scanning electron microscory [J bone Miner Res 2011], courtesy of Dr Jain Q Feng and Dr Lynda F Bonewald).
(B) We generated mice with osteocyte-specific expression of EGFP by crossing CAG-CAT-EGFP reporter mice with a transgenic line expressing Cre recombinase under the control of the Dmp1 promoter (Dmp1-Cre mice) to isolate osteocytes of higher purity. We sorted EGFP-positive and -negative cell populations from the cells obtained by enzymatic digestion of the neonatal calvaria of these mice.
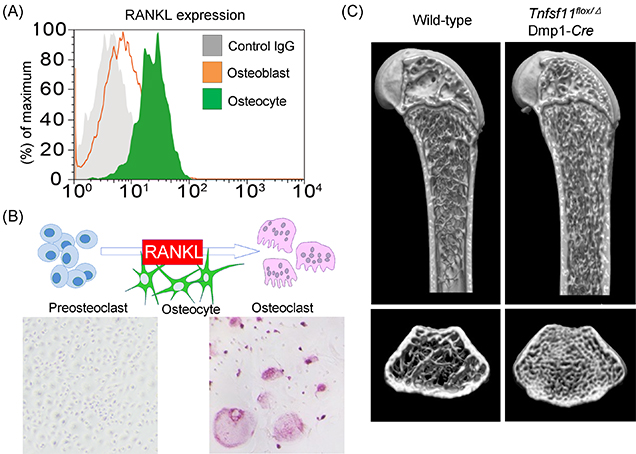
Figure 2: Osteocyte-derived RANKL plays a crucial role in the bone remodeling.
(A) Osteocytes express a much higher amount of RANKL (Flowcytometric analysis).
(B) In vitro osteoclastogenesis was remarkably induced by osteocytes (Tartrate-resistant acid phosphatase [TRAP] staining, TRAP-positive multinuclear cells; osteoclasts).
(C) Osteocyte-specific Tnfsf11-deficient mice (Tnfsf11flox/Δ; Dmp1-Cre mice) exhibited severe osteopetrosis due to a lack of osteoclasts (Microcomputed tomography analysis).
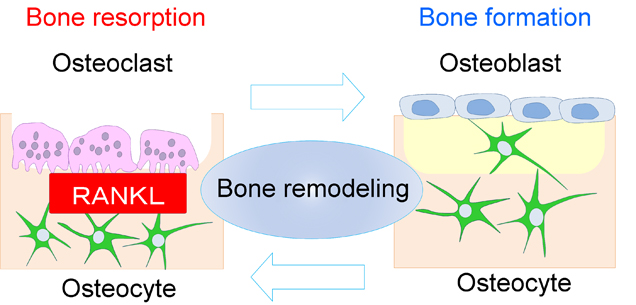
Figure 3: Osteocytes are the commander cell at the initiation of the bone remodeling through RANKL expression.
Osteocytes are thought to orchestrate bone homeostasis by regulating both bone-forming osteoblasts and bone-resorbing osteoclasts, but in vivo evidence and the molecular basis for the regulation has not been sufficiently demonstrated. In the research, we provide in vivo evidence for the key role of osteocyte-derived RANKL in bone remodeling in response to mechanical loading, establishing a molecular basis for osteocyte regulation of bone resorption.

