Expanding the arsenal of drugs against COVID-19
November 2, 2022
Researchers from Tokyo Medical and Dental University (TMDU) have developed novel compounds with potential as drug treatments for COVID-19 by modifying a previous “hit” compound that was active against the SARS-CoV virus
Tokyo, Japan – The ongoing COVID-19 pandemic, caused by the SARS-CoV-2 virus, has been devastating the entire world. While the vaccination program is advancing, drug treatments for COVID-19 are still highly important for those who become infected. Now, a team at Tokyo Medical and Dental University (TMDU), National Center for Global Health and Medicine (NCGM), Tohoku University, NCI/NIH, and Kumamoto University has designed and synthesized compounds that have the potential to be novel drugs targeting SARS-CoV-2.
The SARS-CoV-2 virus contains an enzyme called the “main protease”, or Mpro, that cleaves other proteins encoded in the SARS-CoV-2 genome as part of viral activity and replication. Mpro is an important and appealing target for drugs treating COVID-19 because it is both essential for viral replication and very different from any human molecules, so drugs targeting Mpro are likely to have few side effects and be very effective.
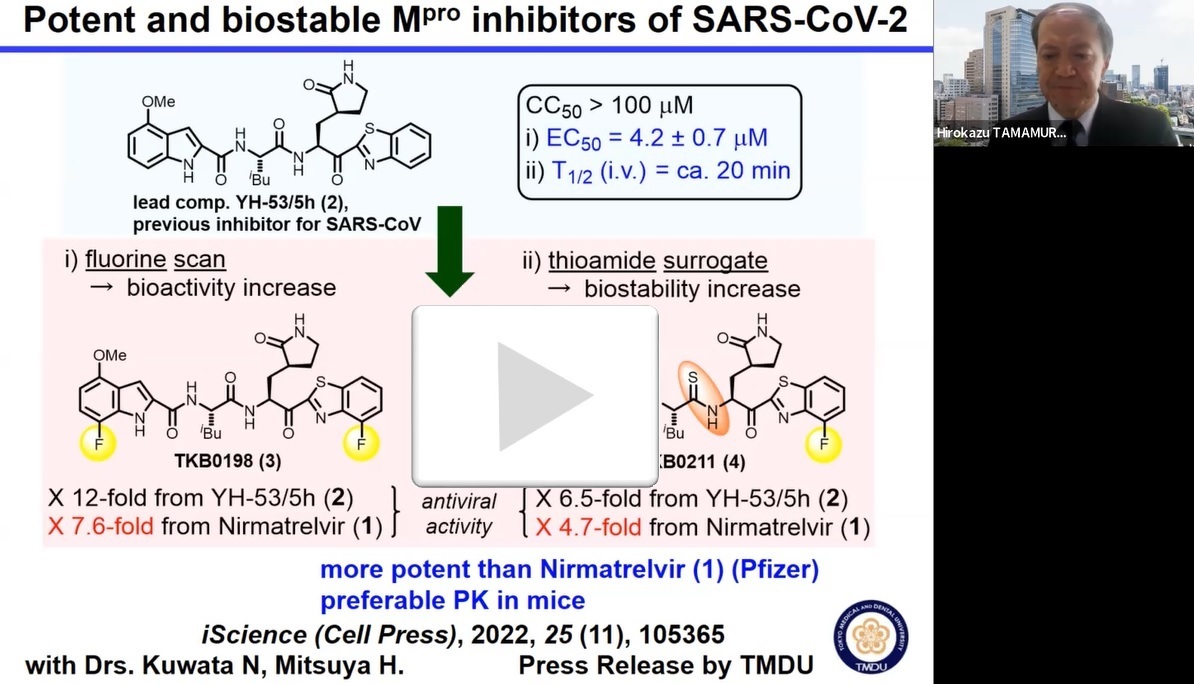
When testing a panel of compounds known to have inhibitory activity against SARS-CoV, the virus responsible for the 2002 SARS outbreak, the team identified a compound named 5h/YH-53 that showed some activity inhibiting SARS-CoV-2 Mpro, but was inefficient and unstable. Therefore, they used 5h as a starting point to develop other compounds with increased efficiency and stability. “Our strategy involved introducing fluorine atoms into the part of the molecule responsible for inhibiting Mpro to increase its binding affinity, as well as replacing a bond within 5h that is easily broken down by the liver with a different structure to increase biostability,” explains lead author Kohei Tsuji.
“Of the compounds we developed, compound 3 showed high potency and was able to block SARS-CoV-2 infection in vitro without any viral breakthrough,” explains senior author Hirokazu Tamamura. “Compound 4, a derivative of compound 3 in which an easily broken-down amide bond had been replaced with a stable thioamide bond, also showed remarkable anti-SARS-CoV-2 activity.” Although compound 4 had lower Mpro inhibitory activity than compound 3, the increased stability meant that the overall activity of compound 4 was comparable to that of compound 3.
When they tested these novel compounds on a variety of strains of SARS-CoV-2, compound 3 was as effective on mutant strains of the virus as on the ancestral Wuhan strain. Additionally, neither compound 3 or 4 showed any toxicity to cultured cells. These data suggest that these compounds show high potential as drug treatments for COVID-19.
A repertory of drug choice is important for treating disease, and so the development of efficient drugs to target the novel SARS-CoV-2 virus is highly important. This work identifies two compounds as potential drugs, and further development of these compounds continues. It also proves the principle that easily broken-down amide bonds can be replaced with thioamide bonds in drug development to increase the stability of the resulting compounds. Taken together, this is an important advance in both the wider drug development field as well as for drugs to treat COVID-19.
The SARS-CoV-2 virus contains an enzyme called the “main protease”, or Mpro, that cleaves other proteins encoded in the SARS-CoV-2 genome as part of viral activity and replication. Mpro is an important and appealing target for drugs treating COVID-19 because it is both essential for viral replication and very different from any human molecules, so drugs targeting Mpro are likely to have few side effects and be very effective.
When testing a panel of compounds known to have inhibitory activity against SARS-CoV, the virus responsible for the 2002 SARS outbreak, the team identified a compound named 5h/YH-53 that showed some activity inhibiting SARS-CoV-2 Mpro, but was inefficient and unstable. Therefore, they used 5h as a starting point to develop other compounds with increased efficiency and stability. “Our strategy involved introducing fluorine atoms into the part of the molecule responsible for inhibiting Mpro to increase its binding affinity, as well as replacing a bond within 5h that is easily broken down by the liver with a different structure to increase biostability,” explains lead author Kohei Tsuji.
“Of the compounds we developed, compound 3 showed high potency and was able to block SARS-CoV-2 infection in vitro without any viral breakthrough,” explains senior author Hirokazu Tamamura. “Compound 4, a derivative of compound 3 in which an easily broken-down amide bond had been replaced with a stable thioamide bond, also showed remarkable anti-SARS-CoV-2 activity.” Although compound 4 had lower Mpro inhibitory activity than compound 3, the increased stability meant that the overall activity of compound 4 was comparable to that of compound 3.
When they tested these novel compounds on a variety of strains of SARS-CoV-2, compound 3 was as effective on mutant strains of the virus as on the ancestral Wuhan strain. Additionally, neither compound 3 or 4 showed any toxicity to cultured cells. These data suggest that these compounds show high potential as drug treatments for COVID-19.
A repertory of drug choice is important for treating disease, and so the development of efficient drugs to target the novel SARS-CoV-2 virus is highly important. This work identifies two compounds as potential drugs, and further development of these compounds continues. It also proves the principle that easily broken-down amide bonds can be replaced with thioamide bonds in drug development to increase the stability of the resulting compounds. Taken together, this is an important advance in both the wider drug development field as well as for drugs to treat COVID-19.
###
The article, “Potent and Biostable Inhibitors of the Main Protease of SARS-CoV-2”, was published in iScience at DOI: 10.1016/j.isci.2022.105365
Summary
Researchers from Tokyo Medical and Dental University (TMDU) have developed novel compounds with the potential to be drug treatments for the SARS-CoV-2 virus, responsible for the COVID-19 pandemic. This work also proves that replacing easily broken-down “amide” bonds with more stable “thioamide” bonds is a viable strategy in drug development to increase the biological stability of a compound. The ongoing development of these compounds will be key in increasing the available drug repertoire for COVID-19 treatment.
Journal Article
JOURNAL:iScience
TITLE:Potent and biostable inhibitors of the main protease of SARS-CoV-2
DOI:https://doi.org/10.1016/j.isci.2022.105365
TITLE:Potent and biostable inhibitors of the main protease of SARS-CoV-2
DOI:https://doi.org/10.1016/j.isci.2022.105365
Correspondence to
Hirokazu TAMAMURA,Ph.D.,Professor
Department of Medicinal Chemistry,
Institute of Biomaterials and Bioengineering,
Tokyo Medical and Dental University(TMDU)
E-mail:tamamura.mr(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.