"Monitoring Thyroid Response: Biomarker May Predict Graves’ Disease Relapse"

Koshi HASHIMOTO, Ph.D.,Associate Professor (Left)
Department of Preemptive Medicine and Metabolism,Graduate School of Medical and Dental Sciences
Yoshihiro OGAWA, Ph.D.,Professor (Right)
Department of Molecular Endocrinology and Metabolism, Graduate School of Medical and Dental Sciences,
Tokyo – Graves’ disease is an autoimmune disorder that leads to overproduction of thyroid hormone. This most common form of hyperthyroidism can cause anxiety, tremors, rapid heartbeat, and, in some patients, a bulging appearance in the eyes. Patients are typically given an anti-thyroid drug for 12–18 months to bring their hormone levels back to normal, but many relapse and experience a hormonal uptick after stopping treatment. Currently, there is no biomarker—a reliable and measurable indicator of disease—that can identify risk of relapse in these patients.
Researchers have now found a gene whose expression is significantly higher in relapsing patients. The gene, SIGLEC1, has the potential to become the first biomarker to predict relapse in Graves’ disease.
In a multicenter study centered at Tokyo Medical and Dental University (TMDU) and recently reported in the journal Thyroid, researchers examined over 400 patients with Graves’ disease, with the hope of finding a marker to predict relapse. At first, however, the investigation began with just two patients.
“We started by looking at differences in gene expression between two patients: one who was in long-term remission and one who suffered from frequent relapses after stopping treatment,” lead author Koshi Hashimoto explains. “We found the relapsing patient exhibited much higher expression of SIGLEC1, a gene known to be involved in autoimmune disorders and possibly in thyroid gland proliferation.”
By initially focusing on only two patients, the researchers were able to conduct a thorough search for potential biomarkers, in this case focusing on gene expression. They used a microarray, which is a chip containing short sequences of DNA that act as microscopic probes, to compare the expression of thousands of genes between the two individuals. SIGLEC1expression was markedly higher in immune cells of the relapsing patient, identifying the gene as a prime biomarker candidate.
Armed with this presumed marker, the research team conducted a cross-sectional study of patients with Graves’ disease who were treated between 2008 and 2015. Compared with those in remission, patients who had previously relapsed showed elevated SIGLEC1 expression at the time of the study (Fig.A). These data allowed the team to group patients as either positive or negative for SIGLEC1—creating a diagnostic cutoff that could theoretically be used to predict future relapse.
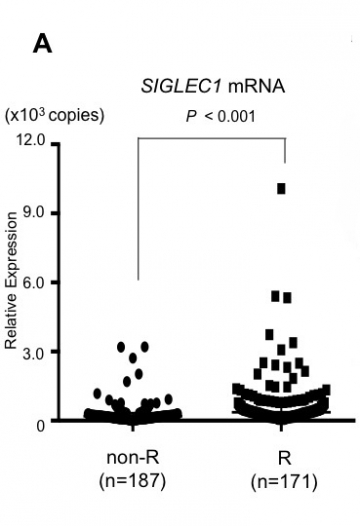
A: High SIGLEC1 mRNA levels in patients with GD relapse experience
SIGLEC1 mRNA levels in peripheral leukocytes are significantly higher in patients with GD relapse experience (R group) than in those who were in long-term remission (non-R group) (cross-sectional study). GD: Graves’ disease.
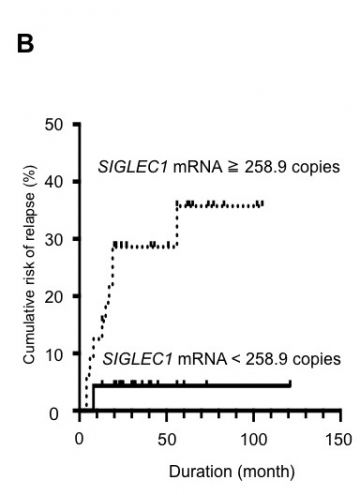
B: SIGLEC1 mRNA levels at ATD discontinuation may predict GD relapse.
SIGLEC1-positive patients (≧258.9 copies) showed a significantly higher cumulative risk for relapse than SIGLEC1-negative patients (< 258.9 copies) (P = 0.022, Hazard Ratio (HR) = 7.508 [95% Confidence Interval (CI): 1.239 to 13.50], the Log-rank test)(prospective study). ATD:anti-thyroid drug.
“The risk of relapse in Graves’ disease is quite high—around 40%–50% in Europe, and 70%–80% in the United States,” co-author Masanobu Yamada notes. “Our finding may give us a tool to find at-risk patients at an earlier stage, and more effectively tailor their treatment options.”
###
The article, “Sialic acid-binding immunoglobulin-like lectin1 as a Novel Predictive Biomarker for Relapse in Graves' Disease -A multicenter study-” was published in Thyroid at DOI: 10.1089/thy.2017.0244.
Summary :
Correspondence to:
Department of Preemptive Medicine and Metabolism,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:khashimoto.mem(at)tmd.ac.jp

