“Tooth regenerative technology by a mechanical ligation method”
summary
Congenital and acquired tooth loss in adults from dental caries, periodontal disease or trauma can be managed using conventional dental treatments involving artificial teeth, such as fixed dental bridges, removable dentures and dental implants. Although these artificial dental therapies have been widely applied to correct tooth loss, further technological improvements based on biological findings are expected to also restore physiological tooth function. Previous studies of autologous tooth germ transplantation, showing that this process prevented immunological rejection after transplantation, have reported successful tooth eruption into the oral cavity and restoration of physiological tooth function. However, the available tooth germs in humans are limited by the number and the developmental stage of individual tooth germs. Based on recent biological findings, we anticipate the development of a novel bioengineering technology that can increase the number of tooth germs.
The team developed a novel mechanical ligation method[1] for splitting tooth germs through spatiotemporal regulation of the reaction-diffusion waves in the tooth-forming field. Each of the split tooth germs developed multiple underlying signalling centres and eventually developed into natural teeth. After transplantation, the split teeth successfully erupted into a region of tooth loss and restored physiological tooth functions, including mastication, periodontal ligament function and appropriate responses to noxious stimuli.
The team demonstrates a new functional tooth regeneration technology utilising split tooth germs through re-regionalisation of the tooth-forming field by applying mechanical force. Further studies to identify tooth germs at the appropriate developmental stage for use and to optimise the splitting procedure in humans are necessary for the future clinical application of split tooth germ transplantation.
Research group
Team Leader Takashi Tsuji
(Tokyo University of Science, Research Institute for Science and Technology, Visiting professor)
Tokyo Medical and Dental University Graduate School of Medical and Dental Science
Department of Maxillofacial Orthognathics
Professor Keiji Moriyama
1.Background
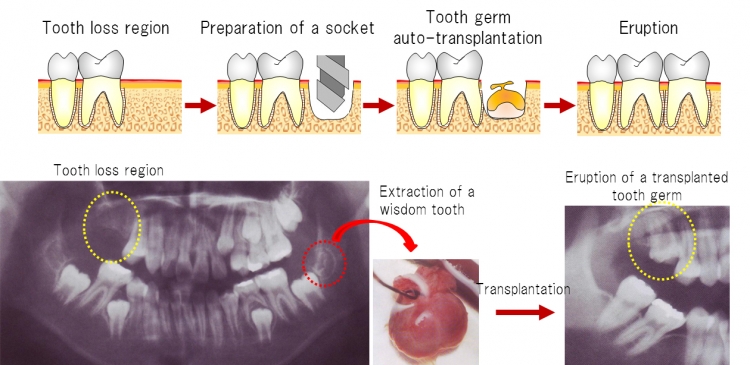
Fig 1 Tooth germ auto-transplantation as a treatment option
Previous studies of autologous tooth germ transplantation, showing that this process prevented immunological rejection after transplantation, have reported successful tooth eruption into the oral cavity and restoration of physiological tooth function.
The team developed a novel mechanical ligation method to increase the number of transplantable tooth germs. Following transplantation, the split tooth germs erupted into tooth-loss regions and restored physiological tooth functions, including mastication, periodontal ligament function through bone remodeling, and proper responsiveness to noxious stimuli. Further studies to splitting germs are necessary for the future clinical application of organ germ transplantation.
2.Achievements
1)Generation of split tooth germs by a mechanical ligation method.
The team dissected molar tooth germs from the mandibles of ED14.5 mice and longitudinally ligated through the central portion using thin nylon thread(Fig 2)Eighty-two per cent of ligated tooth germs (729/889) separated into two tooth germs after 6 days in an in vitro organ culture.
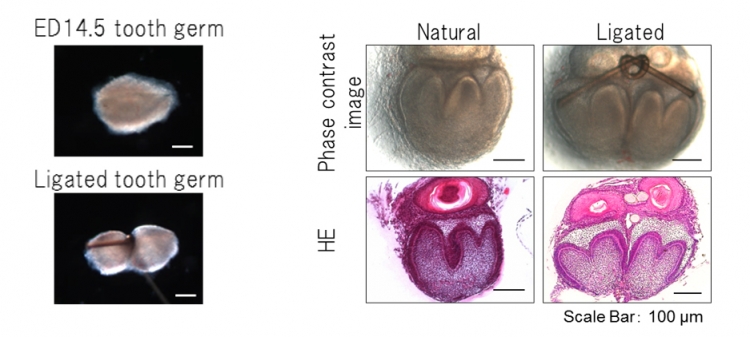
Fig 2 Splitting of a tooth germ by ligation and tissue analysis
Left : A single tooth germ (ED14.5) was longitudinally ligated through the central portion using thin nylon thread. The tooth germ was not completely split into two during this mechanical ligation method.
Right : Phase-contrast images (upper) and HE section images (lower) of ED14.5 ligated tooth germs after day 6 of organ culture. Ligated tooth germs separated into two tooth germs after 6 days in an in vitro organ culture.
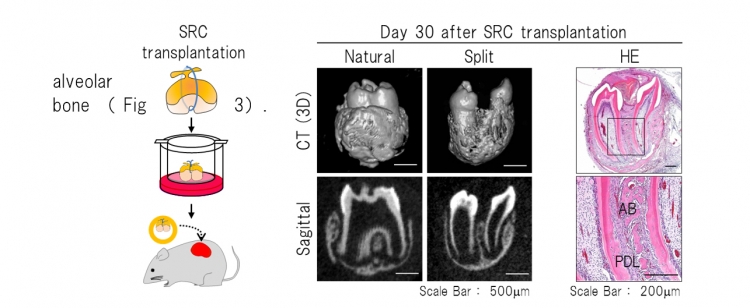
Fig 3 Histological analysis of the split teeth on day 30 after SRC transplantation
Left : Schematic representation of ligation of a tooth germ and transplantation into a SRC.
Right : The split tooth germs generated two teeth with the correct structures of complete molars, and properly formed partitioned periodontal tissue and surrounding alveolar bone. AB: Alveolar bone, PDL: Periodontal ligament
To investigate the developmental process of the split tooth germ, we performed time-lapse live imaging analysis using transgenic mouse embryos to visualize the cell cycle in vivo by monitoring fluorescence in cells in the S/G2/M phases (green nuclei) and those in the G0/G1 phase (red nuclei) (Fig 4). The prospective primary enamel knots, which were labelled in red, were immediately observed in each tooth germ after ligation, and cervical loops at the sides of each split tooth germ gradually developed.
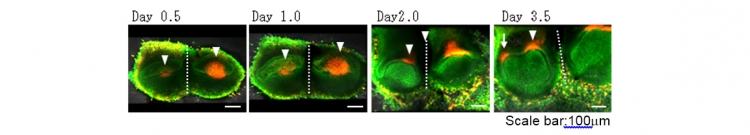
Fig 4 Developmental process of the split tooth germ
Snapshot images of a time-lapse observation of a split tooth germ from a H2B-GFP×Fucci transgenic mouse embryo using a confocal laser microscope.
The team investigated the expression patterns of Shh and Fgf4, which play important roles in the early development of tooth germs, in ED14.5 natural and split tooth germs via in situ hybridization (Fig 5 left). The expression patterns of Shh and Fgf4 mRNAs in the split tooth germ were identical to those in the natural tooth germ. We also investigated the expression pattern of F-spondin (Spondin), which is expressed in dental follicle cells, and of Collagen, type I, alpha 1 (Col1a1), which is expressed in dental mesenchyme and bone (Fig. 5 right). Each split tooth germ developed into separated periodontal tissues after being subjected to mechanical ligation and showed the same gene expression patterns as a natural tooth.
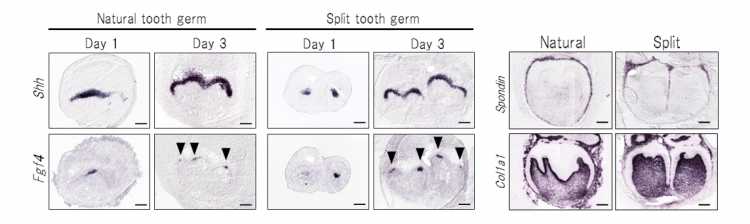
Fig 5 Gene expression patterns of the natural and split tooth germ
Left: In natural and split tooth germs, Shh and Fgf4 mRNA-positive cells were observed in the primary enamel knot after 1-2 days in organ culture. The Shh expression area stretched from the enamel knot to the crown area of the inner enamel epithelium after 3-4 days in organ culture.
Right: After 6 days in organ culture, the expression of Spondin and Col1a1 was detected in the dental follicles and in the dental mesenchyme and bone of the natural tooth germs and each split tooth germ, respectively.
Each split tooth germ developed into separated periodontal tissues after being subjected to mechanical ligation and showed the same gene expression patterns as a natural tooth.
Previous studies have reported that the number of teeth is regulated by the reaction-diffusion waves of the expression of Lef1, an activator, and Ectodin, an inhibitor, in early tooth development. To clarify the expression patterns in the tooth-forming field in split tooth germ development, we analysed the Lef1 and Ectodin expression patterns in ED14.5 natural and split tooth germs by in situ hybridization(Fig 6). After 1 day in organ culture, each split tooth germ exhibited both a Lef1-expressing activation area and an Ectodin-expressing inhibition area surrounding the Lef1-expressing area. In addition, the gene expression intensities (shown in Fig 5 right) indicate that the period of the gene expression pattern of split tooth germs is half that of natural tooth germs. These findings suggest that the tooth number and crown size are determined by the balance of expression of a specific activator and inhibitor and that they are molded by the re-regionalization of reaction-diffusion waves in the tooth-forming field.
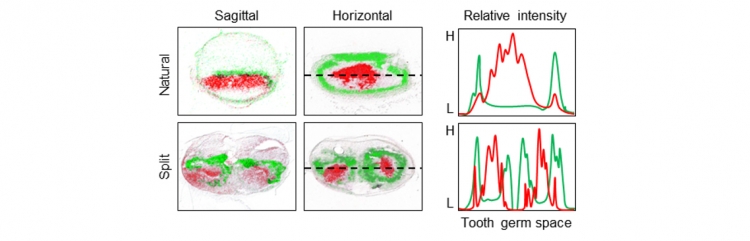
Fig 6:The gene expression patterns of Lef1 and Ectodin
False collar overlays of Lef1 (red) and Ectodin (green) expression patterns in ED14.5 natural tooth germs (upper) and split tooth germs (lower) in sagittal (left) and horizontal (centre) sections. The gene expression intensities (right) indicate that the period of the gene expression pattern of split tooth germs is half that of natural tooth germs.
The team next investigated whether a split tooth germ, ligated with an 8-0 nylon thread at ED14.5, erupted and became engrafted after transplantation into a tooth loss region. After 4-6 days in organ culture, a split tooth germ was transplanted in the correct orientation into a prepared socket of the upper first molar alveolar region in an adult murine tooth-loss transplantation model. Two months after transplantation, 71.9% of the split teeth (110/153) had formed the correct structures and also had reached occlusal contact with the opposing mandibular first molar (Fig 7).
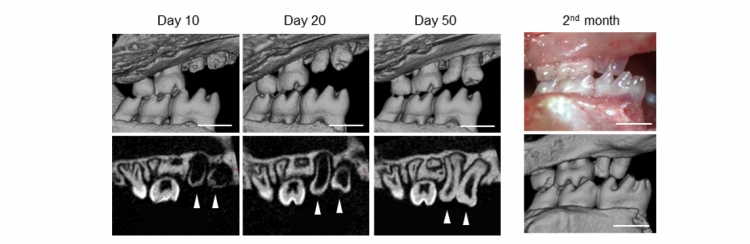
Fig 7 Eruption and occlusion of split teeth
The split teeth formed the correct structures and reached the occlusal plane two months after transplantation
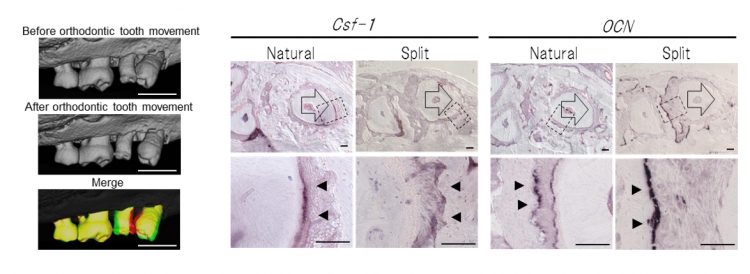
Fig 8 Orthodontic tooth movement of split teeth
Left: Micro-CT images before (upper) and after (lower) orthodontic tooth movement, and superposition of micro-CT images at day 0 (red) and day 6 (green) of orthodontic tooth movement.
Right: Ocn mRNA-positive cells (arrowhead) and Csf-1 mRNA-positive cells (arrow) are indicated on day 6 of orthodontic movement.
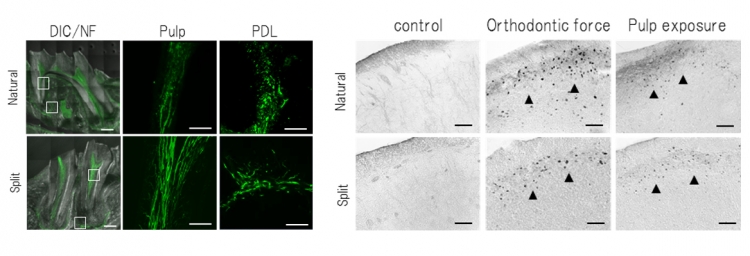
Fig 9 Analysis of nerve fibers and c-Fos-immunoreactivity
Left:Neurofilament (NF)-immunoreactive nerve fibres were detected in the pulp and the PDL of the natural and engrafted split teeth.
Right:c-Fos was observed in both the natural and split teeth 2 hours after stimulation
3.Future prospect
In addition, our new technology could contribute to the elucidation of tooth germ formation and the determination of tooth size.
4.Article information
Functional tooth restoration utilising split germs through re-regionalisation of the tooth-forming field
<Author>
Naomi Yamamoto, Masamitsu Oshima, Chie Tanaka, Miho Ogawa, Kei Nakajima, Kentaro Ishida, Keiji Moriyama & Takashi Tsuji
<journal>
Scientific Reports
<DOI>
10.1038/srep18393
5.Additional explanation
To split germs by ligating to artificially regulate the molecules that control organ morphology.
[2] bone remodeling
Bone remodeling is an active and dynamic process that relies on the correct balance between bone resorption by osteoclasts and bone deposition by osteoblasts. Tooth movement by orthodontic force is dependent on efficient remodeling in periodontal ligament and alveolar bone.
[3] enamel knot
The enamel knot represents a signal center for tooth morphogenesis. Tooth cusp formation commences with the appearance of the enamel knot, which is a cluster of non-dividing epithelial cells.
[4] H2B-GFPtransgenic mice
The transgenic mice which express the human histone H2B gene and the green fluorescent protein (GFP) fusion protein.
[5] cell cycle
The cell cycle is the series of events that take place in a cell division. The cell cycle can be divided into four periods: Cells increase in size in G1. DNA replication occurs in S. The cell will continue to grow in G2. Cell growth stops and all of the cell's energy is focused on division into two similar daughter cells at M.
[6] reaction-diffusion model
The mechanisms of morphogenesis can be explained by ``reaction-diffusion'' equations. It has been reported that periodic patterns emerge in many cases during development (such as the skin pigment pattern in zebra, zebrafish and sea shells, feather follicle formation, tooth development and digit formation during limb development).
[7] medullary dorsal horn
The medullary dorsal horn is a site for processing orofacial pain. In the medullary dorsal horn, the levels of c-Fos immunoreactivity increased significantly after acute noxious stimulation by inflammation in the orofacial regions.
6.Contact information
RIKEN Center for Developmental Biology Laboratory for Organ Regeneration
Team Leader Takashi Tsuji
TEL:078-306-3447 FAX:078-306-3449
E-mail:t-tsuji(at)cdb.riken.jp
Tokyo Medical and Dental University Graduate School of Medical and Dental Science
Department of Maxillofacial Orthognathics
Professor Keiji Moriyama
E-mail:k-moriyama.mort(at)tmd.ac.jp
TEL: 03-5803-5532
<Inquiries>
RIKEN Public relations office
TEL:048-467-9272 FAX:048-462-4715
E-mail:ex-press(at)riken.jp
Tokyo Medical and Dental University
TEL:03-5803-5833 FAX:03-5803-0272
E-mail:kouhou.adm(at)tmd.ac.jp
*Please change (at) in the e-mail addresses to @ when sending correspondence.

