”Molecule’s Role in Maintaining Liver Size and Function Revealed”
Tokyo, Japan – For organs to maintain a steady state and fulfill their intended functions, the rates at which the cells within them multiply or die off need to be equal. This balance must also be adjusted when events such as an injury or infection occur. However, the mechanisms by which such balance is achieved have remained obscure, including for the liver, which is particularly vulnerable to destabilization owing to its function in detoxifying the body.
In a major advance on this issue, a Tokyo Medical and Dental University (TMDU)-led team has shown that a molecule called YAP regulates the fate of liver cells called hepatocytes, determining whether they proliferate to boost the organ’s bulk or are degraded and removed. This choice was shown to be dependent on whether the liver cells had been damaged, which deepens our understanding of how this organ maintains itself.
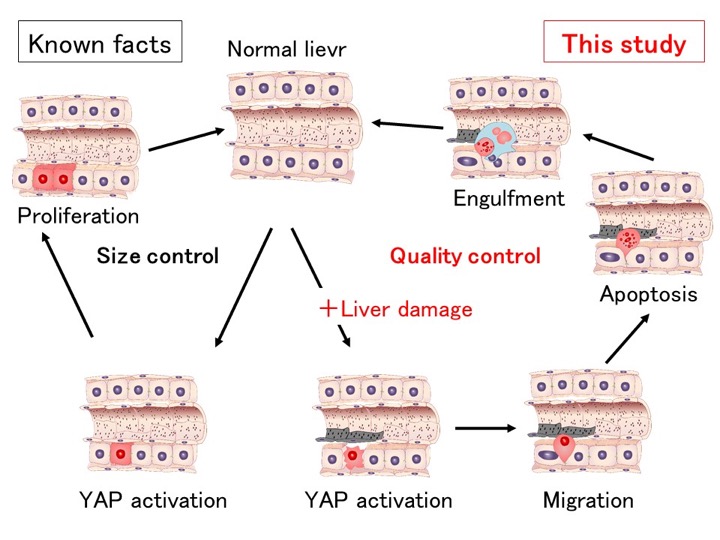
Fig: YAP-dependent liver size and quality controls
Active YAP regulates the liver size through hepatocyte proliferation (left; known facts). In this study, we showed that active YAP selectively eliminates damaged hepatocytes (right). Hepatocytes expressing activated YAP in the presence of liver injury such as ethanol migrate into sinusoids, undergo apoptosis and are engulfed by Kupffer cells. Thus, a change in hepatocyte fate from proliferation to migration/apoptosis depends on a mechanism of stress detection involving YAP.
“Once we suspected that cell injury was a key factor determining which effect YAP had on the liver cell population, we analyzed which type of liver injury caused YAP to induce cells to be eliminated rather than multiply using toxic chemicals that damage only specific liver cell types,” says Hiroshi Nishina of the Department of Developmental and Regenerative Biology, TMDU. “We found that damage to both hepatocytes and cells called liver sinusoidal endothelial cells led to the induction of hepatocyte elimination by YAP, which provided a strong hint about the process behind this elimination.”
Specifically, the team showed that YAP is activated upon damage to cells in the liver, leading to the migration of these cells to special blood vessels in the liver. There, they undergo apoptotic cell death and are engulfed and degraded by Kupffer cells, which are liver-specific immune cells.
“This shows how effectively the liver can regulate its own size and function,” co-author Norio Miyamura says. “Without regulatory mechanisms like this, the liver would be much more susceptible to becoming enlarged or developing cancerous growths.”
The obtained findings could also help explain the causes of liver diseases associated with dysregulation of some of the key processes that enable this organ to function.
The article “YAP determines the cell fate of injured mouse hepatocytes in vivo” was published in Nature Communications at doi: 10.1038/ncomms16017.
Summary Text:
Correspondence to:
Department of Developmental and Regenerative Biology,
Medical Research Institute,
Tokyo Medical and Dental University (TMDU)
Phone:+81-3-5803-4659 Fax:+81-3-5803-0272
E-mail: nishina.dbio (at) mri.tmd.ac.jp

