"Forming and resorbing bones: metastasizing cancer cells modify bone remodeling with small RNA secretion"

Shingo Sato,PhD, Junior Associate Professor
Department of Physiology and Cell Biology
Researchers at Tokyo Medical and Dental University (TMDU) find a secreted microRNA molecule that alters bone structure in late-stage prostate cancer
Tokyo – Prostate cancer is one of the most common cancers in men worldwide. In nearly three out of four patients the cancer causes metastasis, leaving the original site of the tumor and spreading to distant sites in the body. Metastatic prostate cancer cells often travel to bone, where they can affect bone structure and cause severe pain, pathological fractures, and spinal cord compression. A better understanding of the process that enables these cancer cells to remodel bone may aid in the search for new therapies. In a study published in Proceedings of the National Academy of Sciences, researchers at Tokyo Medical and Dental University (TMDU) report the discovery of an RNA molecule that may play a key role in bone restructuring caused by prostate cancer (Fig.1).
Tokyo – Prostate cancer is one of the most common cancers in men worldwide. In nearly three out of four patients the cancer causes metastasis, leaving the original site of the tumor and spreading to distant sites in the body. Metastatic prostate cancer cells often travel to bone, where they can affect bone structure and cause severe pain, pathological fractures, and spinal cord compression. A better understanding of the process that enables these cancer cells to remodel bone may aid in the search for new therapies. In a study published in Proceedings of the National Academy of Sciences, researchers at Tokyo Medical and Dental University (TMDU) report the discovery of an RNA molecule that may play a key role in bone restructuring caused by prostate cancer (Fig.1).
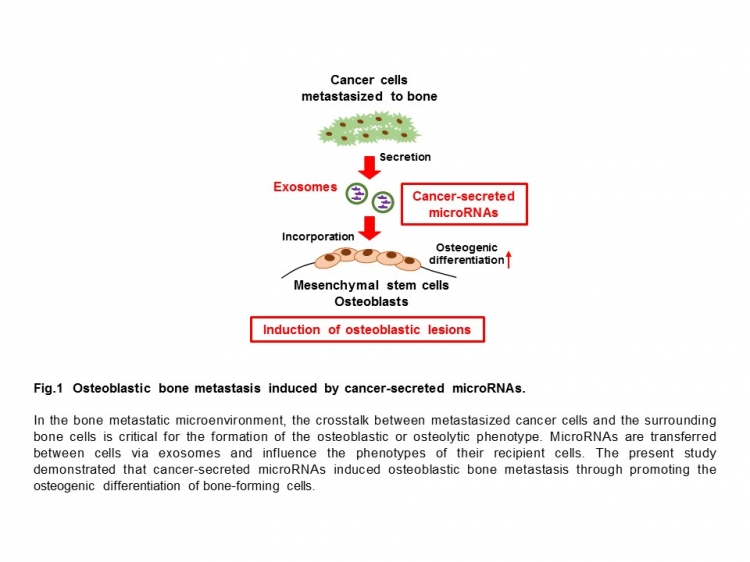
Proper bone development is maintained through a fine balance of bone-forming (osteoblast) and bone-resorbing (osteoclast) cells. Tumor cells metastasized to bone modify the activity of these cells and, depending on the type of cancer, cause osteoblastic or osteolytic changes.
“Metastatic tumor cells can promote either bone formation or bone resorption,” lead author Kyoko Hashimoto explains. “Osteoblastic lesions are bone-forming sites that are commonly found in prostate cancer. These lesions cause structural weakness in the bone and, even worse, promote additional tumor growth. If we can figure out how prostate cancer cells alter the environment inside bone to induce this osteoblastic process, we can start to identify new therapeutic targets.”
To accomplish this, the researchers looked for differences between bone-forming prostate cancer cells and bone-resorbing breast cancer cells. They focused on microRNAs—short RNA molecules that regulate the expression of a variety of genes. Recent studies have shown that microRNAs are delivered from cells to cells via exosomes to serve as intercellular communication tools. MicroRNAs secreted by cancer cells can alter the expression of genes in non-cancerous cells, disrupting their normal function and promoting tumor growth.
The team looked at a panel of microRNAs and compared their abundance in exosomes derived from prostate cancer and breast cancer. One microRNA in particular, miR-940, was highly abundant in the prostate cancer-derived exosomes and turned out to play a powerful role in promoting bone formation.
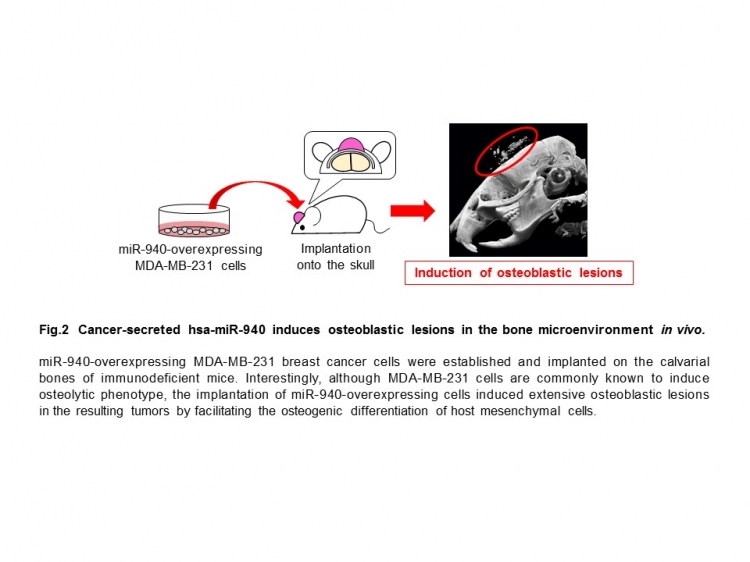
“We found that miR-940 can be secreted from metastatic cancer cells and alter gene expression in cells that reside in bone, causing them to take on osteoblast-like properties,” Hashimoto adds. “When implanted into the tibia or onto the skull of mice, moreover, we discovered that cells expressing miR-940 can induce the kind of osteoblastic lesions that are typical of prostate cancer.”
Strikingly, when miR-940 was expressed in bone-resorbing breast cancer cells, the cells triggered bone-forming lesions in mice—behaving as though they were prostate cancer cells (Fig.2). Their findings suggest that miR-940 plays a central role in the bone-forming ability of prostate cancer.
“Metastatic tumor cells can promote either bone formation or bone resorption,” lead author Kyoko Hashimoto explains. “Osteoblastic lesions are bone-forming sites that are commonly found in prostate cancer. These lesions cause structural weakness in the bone and, even worse, promote additional tumor growth. If we can figure out how prostate cancer cells alter the environment inside bone to induce this osteoblastic process, we can start to identify new therapeutic targets.”
To accomplish this, the researchers looked for differences between bone-forming prostate cancer cells and bone-resorbing breast cancer cells. They focused on microRNAs—short RNA molecules that regulate the expression of a variety of genes. Recent studies have shown that microRNAs are delivered from cells to cells via exosomes to serve as intercellular communication tools. MicroRNAs secreted by cancer cells can alter the expression of genes in non-cancerous cells, disrupting their normal function and promoting tumor growth.
The team looked at a panel of microRNAs and compared their abundance in exosomes derived from prostate cancer and breast cancer. One microRNA in particular, miR-940, was highly abundant in the prostate cancer-derived exosomes and turned out to play a powerful role in promoting bone formation.
“We found that miR-940 can be secreted from metastatic cancer cells and alter gene expression in cells that reside in bone, causing them to take on osteoblast-like properties,” Hashimoto adds. “When implanted into the tibia or onto the skull of mice, moreover, we discovered that cells expressing miR-940 can induce the kind of osteoblastic lesions that are typical of prostate cancer.”
Strikingly, when miR-940 was expressed in bone-resorbing breast cancer cells, the cells triggered bone-forming lesions in mice—behaving as though they were prostate cancer cells (Fig.2). Their findings suggest that miR-940 plays a central role in the bone-forming ability of prostate cancer.
“Our study suggests that microRNAs are an important factor that modify bone remodeling and promote tumor growth in metastatic prostate cancer,” lead investigator Shingo Sato concludes. “Once cancer has metastasized to bone it is usually incurable, so any insight into the process of metastasis is immensely helpful in the search for novel therapies. We’re hopeful that our findings will eventually open a new pathway for therapeutic targets in this form of cancer.”
###
The article, “Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A,” was published in Proceedings of the National Academy of Sciences at DOI: 10.1073/pnas.1717363115.
###
The article, “Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A,” was published in Proceedings of the National Academy of Sciences at DOI: 10.1073/pnas.1717363115.
Summary:
Prostate cancer frequently metastasizes and spreads to bone, causing severe pain, fractures, and other complications. The mechanisms that allow cancer cells to modify bone remodeling are incompletely understood. Researchers at Tokyo Medical and Dental University (TMDU) identified a microRNA, miR-940, that is abundant in exosomes secreted by prostate cancer cells and can trigger bone-forming lesions in mice. The findings indicate that microRNAs play a key role in bone metastatic environment and suggest potential novel therapeutic targets for bone metastasis.
Correspondence to:
Shingo Sato,MD,PhD, Junior Associate Professor
Department of Physiology and Cell Biology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:satoshin.phy2(at)tmd.ac.jp
Department of Physiology and Cell Biology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:satoshin.phy2(at)tmd.ac.jp

