Promotion of Cancer Progression Via Extracellular Vesicles
Researchers led by Tokyo Medical and Dental University (TMDU) investigate the role of extracellular vesicles secreted by cancer cells in the promotion of vascular destabilization
Tokyo, Japan – The advent of cell phones, the internet, and various messaging platforms has allowed for faster and broader communication worldwide. But did you know that your body has its own complex communication system in the form of extracellular vesicles (EVs)? These small structures, which contain cellular “cargo” such as protein and nucleic acids, are secreted by cells and can travel throughout the body, influencing a variety of physiological and pathological processes. Recently, researchers in Japan have shed new light on the role of EVs in the progression of cancer.
In a new study published in Inflammation and Regeneration, researchers led by Tokyo Medical and Dental University (TMDU) examined the effects of oral cancer cell-derived EVs in endothelial-mesenchymal transition (EndoMT). EndoMT is a process in which endothelial cells, or cells that line the blood vessels, lose their characteristics and take on properties of mesenchymal cells.
While EndoMT occurs normally during embryonic development, it has also been shown to destabilize vascular structures such as blood vessels. In cancer, vascular destabilization may make it easier for cancer cells to enter and exit the bloodstream, thereby promoting metastasis. Previous research has shown that cancer cells release EVs that act to induce a similar process called epithelial-mesenchymal transition (EMT), thereby promoting tumor development. However, the effects of cancer cell EVs on EndoMT in normal vascular endothelial cells have not yet been elucidated. Therefore, the TMDU-led research team set out to characterize EVs released by oral cancer cells and to investigate the effects of these EVs on healthy vascular endothelial cells.
“A signaling factor known as transforming growth factor-β (TGF-β) has been shown to induce EMT in cancer cells, so we began by investigating charactered changes of human oral cancer cells brought by TGF-β-induced EMT” says lead author Miho Kobayashi. “We found that oral cancer cells exposed to TGF-β released roughly three times as many EVs as those that were not exposed.”
The research team next incubated human vascular endothelial cells with EVs from TGF-β-exposed cells or EVs from cells that were not exposed to TGF-β. RNA and protein analysis showed increased expression of mesenchymal cell markers and decreased expression of endothelial cell markers in vascular cells exposed to EVs secreted from cancer cells that were exposed to TGF-β.
“The changes in endothelial and mesenchymal marker expression in the vascular cells indicated that EndoMT was induced by EVs from oral cancer cells in which EMT had occurred,” says lead author Kashio Fujiwara. Furthermore, evaluation of the effects of cancer cell-derived EVs on vascular stability in human vascular endothelial monolayer cultures showed that treatment with the EVs enhanced vascular destabilization.
This research provides further understanding of the mechanisms involved in EndoMT and vascular instability, which may aid in the development of therapeutic treatments to disrupt cancer progression and metastasis.
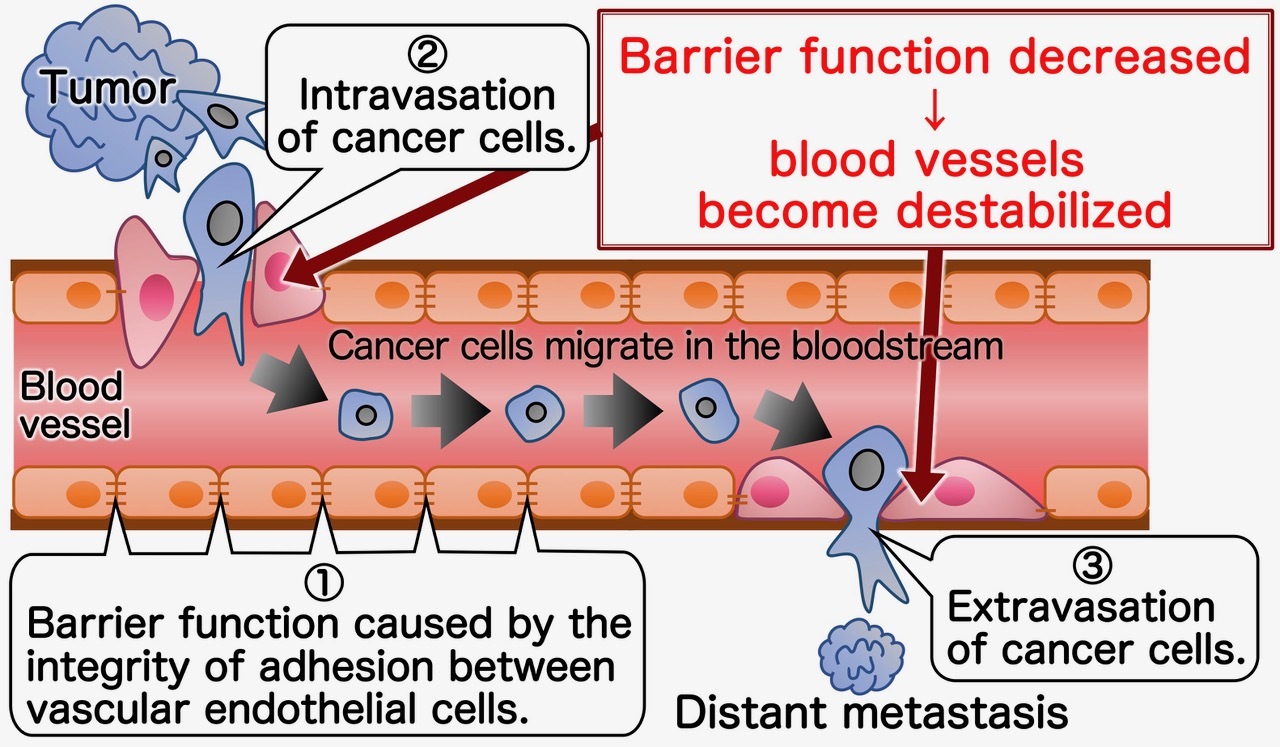
Figure 1. Mechanism of distant metastasis using blood vessels as a migration route
Usually, blood vessels are stabilized, and their barrier function is maintained by the tight adhesion between vascular endothelial cells (①). When cancer cells metastasize to distant organs via blood vessels, the morphology of vascular endothelial cells changes in response to the growth factors or cytokines secreted by the cancer cells. This results in the loss of adhesion between vascular endothelial, destabilization of the vessels and loss of their barrier function. These changes facilitate invasion, “intravasation" (②) of cancer cells into blood vessels and allow the cancer cells to travel through the body in the bloodstream. When cancer cells reach the distant organs, they migrate out of the blood vessels in a process called "extravasation" (③) by inducing vascular destabilization, followed by formation of distant metastatic sites.
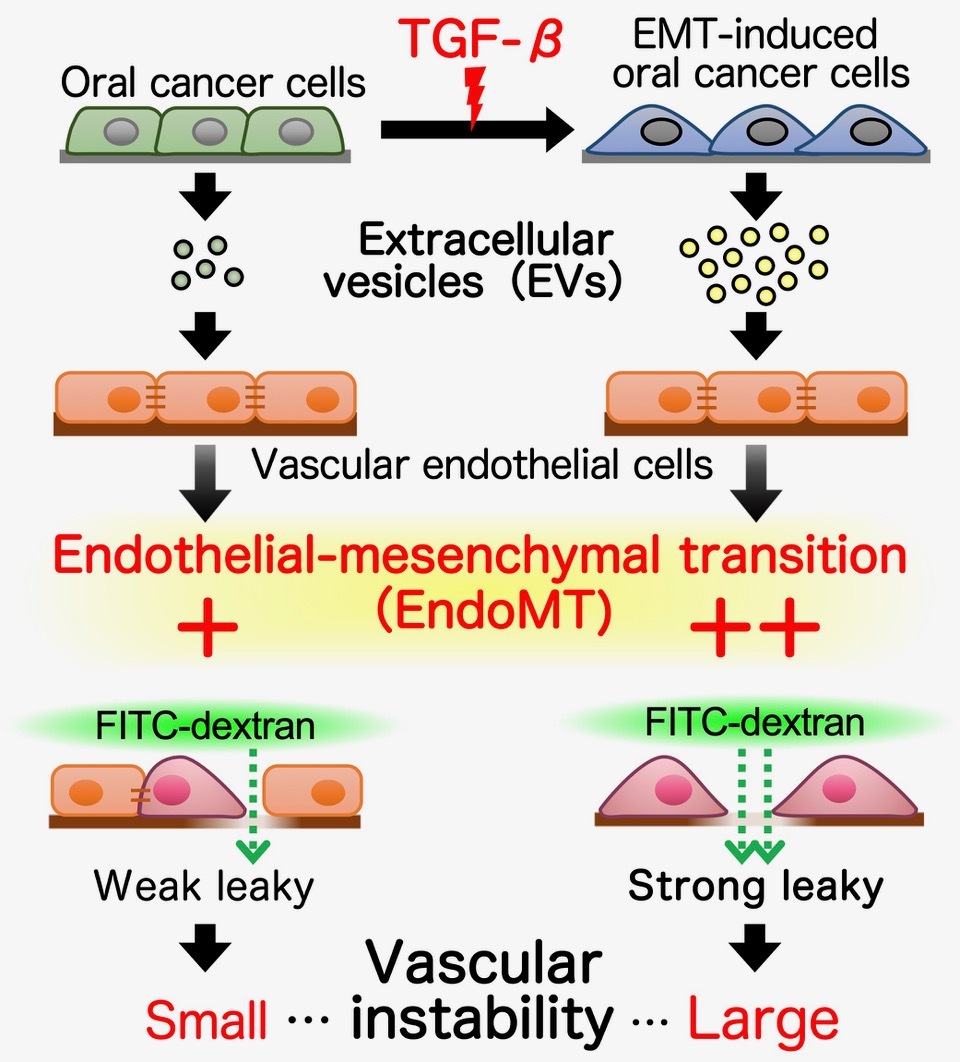
Figure 2. Mechanism of vascular destabilization by TGF-β-induced EndoMT through extracellular vesicles (EVs)
In exacerbated oral cancer cells via TGF-β-stimulated epithelial-mesenchymal transition (EMT), the amount of secreted EVs increased as compared to unstimulated oral cancer cells. TGF-β-stimulated oral cancer cell-derived EVs induced endothelial mesenchymal transition (EndoMT) in normal vascular endothelial cells to higher extent if compared with effect induced by EVs from non-stimulated oral cancer cells. In addition, the treatment of vascular endothelial sheets formed by endothelial cells with these EVs induced the formation of gaps in vascular endothelial sheet leading to increased permeability. Our results suggest that TGF-β-stimulated EMT-induced oral cancer cells contribute to cancer metastasis by secreting EVs that induce EndoMT in vascular endothelial cells and enhance vascular destabilization.
The article, “Transforming growth factor-β-induced secretion of extracellular vesicles from oral cancer cells evokes endothelial barrier instability via endothelial-mesenchymal transition,” was published in Inflammation and Regeneration at DOI: 10.1186/s41232-022-00225-7
Summary
Journal Article
TITLE:Transforming growth factor-β-induced secretion of extracellular vesicles from oral cancer cells evokes endothelial barrier instability via endothelial-mesenchymal transition
DOI:https://doi.org/10.1186/s41232-022-00225-7
Correspondence to
Miho Kobayashi, Assistant Professor
Katarzyna A. Podyma-Inoue, Assistant Professor
Tetsuro Watabe, Professor
Department of Biochemistry,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:t-watabe.bch(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

