"Faulty sensing: cellular energy sensor linked to the progression of chronic kidney disease"
Researchers at Tokyo Medical and Dental University (TMDU) and Kyushu University find that failure of a critical cellular energy sensor is responsible for progression of chronic kidney disease and may represent a novel therapeutic target
Tokyo, Japan – Chronic kidney disease (CKD), an affliction characterized by progressive loss of kidney function, affects millions of people worldwide and is associated with multi-organ damage, cardiovascular disease, and muscle wasting. Just like engines, living cells require energy to run, thus the combined millions of cells forming an organ have huge energy requirements. Although the heart has the highest energy needs of all human organs, the kidneys come a close second. Energy depletion can result in kidney damage and the build-up of toxic compounds in the body, contributing to the progression of CKD. Currently, there is no effective treatment to halt this progression.
Adenosine triphosphate (ATP) is the major “fuel” in most living cells and is converted to adenosine monophosphate (AMP) during energy transfer. A specialized energy sensor called 5ʹ-AMP-activated protein kinase (AMPK) detects even the slightest changes in cellular energy by sensing AMP levels, triggering the production of ATP in response to energy depletion. However, AMPK activity is decreased in CKD and the mechanism controlling this dysregulation is unclear.
Now, a research team from TMDU and Kyushu University has shown that failure to sense AMP is the key mechanism underlying the inactivity of AMPK in CKD. In a recent study published in Kidney International, they outline how they came to this conclusion and what it may mean for CKD patients.
Tokyo, Japan – Chronic kidney disease (CKD), an affliction characterized by progressive loss of kidney function, affects millions of people worldwide and is associated with multi-organ damage, cardiovascular disease, and muscle wasting. Just like engines, living cells require energy to run, thus the combined millions of cells forming an organ have huge energy requirements. Although the heart has the highest energy needs of all human organs, the kidneys come a close second. Energy depletion can result in kidney damage and the build-up of toxic compounds in the body, contributing to the progression of CKD. Currently, there is no effective treatment to halt this progression.
Adenosine triphosphate (ATP) is the major “fuel” in most living cells and is converted to adenosine monophosphate (AMP) during energy transfer. A specialized energy sensor called 5ʹ-AMP-activated protein kinase (AMPK) detects even the slightest changes in cellular energy by sensing AMP levels, triggering the production of ATP in response to energy depletion. However, AMPK activity is decreased in CKD and the mechanism controlling this dysregulation is unclear.
Now, a research team from TMDU and Kyushu University has shown that failure to sense AMP is the key mechanism underlying the inactivity of AMPK in CKD. In a recent study published in Kidney International, they outline how they came to this conclusion and what it may mean for CKD patients.
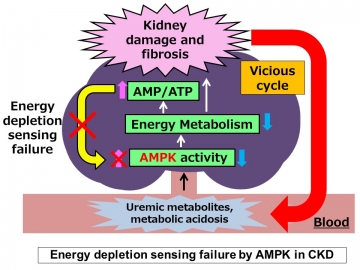
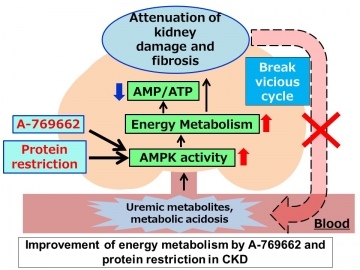
In a mouse model of CKD, metabolome analysis confirmed a decrease in AMPK activation in the kidneys despite a high AMP: ATP ratio, suggesting that AMP K did not sense energy depletion. Several uremic factors were shown to inactivate AMP K in vitro and in ex vivo preparations of kidney tissue. The specific AMPK activator A-769662, which bypasses the AMP sensing mechanism, ameliorated fibrosis and improved energy status in the kidneys of CKD mice, whereas an AMP analog did not. We further demonstrated that a low-protein diet activated AMPK independent of the AMP sensing mechanism, leading to improvement in energy metabolism and kidney fibrosis.
“Metabolites can tell us a lot about what’s going on in a cell,” explains lead author Hiroaki Kikuchi. “In CKD mice, metabolite profiling showed that despite high levels of AMP, there was a substantial decrease in AMPK activation, leading us to conclude that the AMP-sensing function of AMPK was defective.”
Armed with this new information, the researchers tried bypassing the AMP-sensing mechanism to determine whether AMPK could still be activated in CKD mice. By treating the mice with A-769662, an AMPK activator that binds at a different site to AMP, they could significantly attenuate CKD progression and correct associated tissue damage.
Critically, the build-up of waste products in the blood as a result of reduced kidney function was shown to be responsible for the decreased AMP-sensing activity of AMPK. “Our findings suggest that energy depletion, CKD progression, and the accumulation of toxic metabolites form a vicious cycle in CKD patients,” says co-corresponding author Eisei Sohara. “However, AMPK activation via AMP-independent mechanisms can break this cycle and represents a novel therapeutic approach for the treatment of CKD.”
The article, “Failure to sense energy depletion may be a novel therapeutic target in chronic kidney disease”, was published in Kidney International at https://doi.org/10.1016/ j.kint.2018.08.030.
Armed with this new information, the researchers tried bypassing the AMP-sensing mechanism to determine whether AMPK could still be activated in CKD mice. By treating the mice with A-769662, an AMPK activator that binds at a different site to AMP, they could significantly attenuate CKD progression and correct associated tissue damage.
Critically, the build-up of waste products in the blood as a result of reduced kidney function was shown to be responsible for the decreased AMP-sensing activity of AMPK. “Our findings suggest that energy depletion, CKD progression, and the accumulation of toxic metabolites form a vicious cycle in CKD patients,” says co-corresponding author Eisei Sohara. “However, AMPK activation via AMP-independent mechanisms can break this cycle and represents a novel therapeutic approach for the treatment of CKD.”
The article, “Failure to sense energy depletion may be a novel therapeutic target in chronic kidney disease”, was published in Kidney International at https://doi.org/10.1016/ j.kint.2018.08.030.
Summary:
Chronic kidney disease (CKD) is associated with energy depletion in kidney cells, resulting in reduced kidney function. Researchers at TMDU and Kyushu University found that failure of an important cellular energy sensor to detect energy depletion is key to the progression of CKD. However, by stimulating the activity of the sensor using alternative methods, the researchers could halt CKD progression and repair some of the tissue damage. This mechanism therefore represents a novel therapeutic target.
Correspondence to:
Eisei SOHARA,M.D.,Ph.D.,Associate Professor
Department of Nephrology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:esohara.kid(at)tmd.ac.jp
Shinichi UCHIDA,M.D.,Ph.D.,Professor
Department of Nephrology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:suchida.kid(at)tmd.ac.jp
Department of Nephrology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:esohara.kid(at)tmd.ac.jp
Shinichi UCHIDA,M.D.,Ph.D.,Professor
Department of Nephrology,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail:suchida.kid(at)tmd.ac.jp

