Taking Out the Trash is Essential for Brain Health
Tokyo, Japan – A little mess never killed anyone, right? Wrong. Researchers at Tokyo Medical and Dental University (TMDU) have recently shown that a build-up of cellular “trash” in the brain can actually cause neurodegeneration, and even death.
Reporting their findings in Nature Communications, the researchers describe how defects in a cellular waste disposal mechanism, called “alternative autophagy”, can lead to a lethal build-up of iron and protein in brain cells.
“Cells are constantly clearing out dysfunctional or unnecessary components, which are then degraded and recycled,” explains study lead author Hirofumi Yamaguchi. “Autophagy is the process whereby unwanted cellular components and proteins are contained within a spherical doubled-membraned vesicle called an autophagosome, which fuses with an enzyme-filled lysosome to form an autolysosome. The waste material is then broken down and reused by the cell.”
This common form of autophagy, called “canonical autophagy”, is well characterized and involves a suite of autophagy-related proteins, such as Atg5 and Atg7. More recently though, several Atg5-independent alternative autophagy pathways have also been described, the biological roles of which remain unclear.
After identifying alternative autophagy-related proteins in yeast, the team at TMDU focused on a mammalian ortholog called “Wipi3”, which had previously been implicated in canonical autophagy. “When we deleted Wipi3 in a mouse cell line and induced alternative autophagy, we no longer observed the formation of double-membraned autophagosomes or single-membraned autolysosomes, confirming that Wipi3 is essential for alternative autophagy,” says Yamaguchi.
Mice containing a brain-specific deletion of Wipi3 demonstrated growth and motor defects most commonly seen in patients with neurodegeneration, with the researchers also noting an accumulation of iron and the iron-metabolizing protein ceruloplasmin in the brain cells of affected mice.
“Iron deposition has been flagged as a possible trigger in various neurodegenerative disorders, and is usually associated with the abnormal accumulation of iron-binding proteins,” explains study senior author Shigeomi Shimizu. “Our findings are strong evidence that alternative autophagy, and Wipi3 specifically, may be essential for preventing this toxic build-up of iron.”
Interestingly, although Wipi3-deficient and Atg7 (canonical autophagy)-deficient mice showed similar motor defects, they exhibited very different sub-cellular changes, suggesting that alternative autophagy and canonical autophagy act independently to protect neurons. Supporting this, deletion of both Wipi3 and Atg7 in mice was almost always fatal.
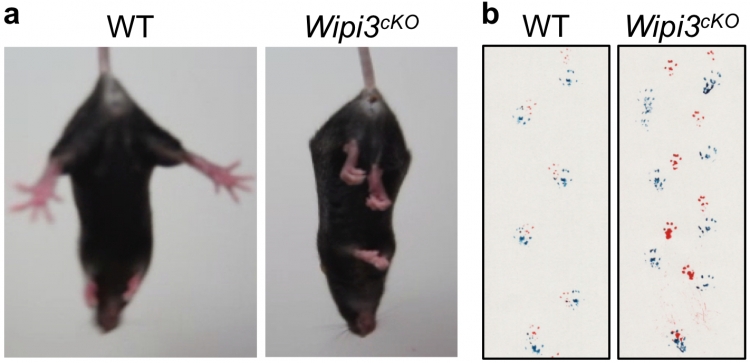
Neurological defects in neuron-specific Wipi3cKO mice
Abnormal motor performance in Wipi3cKO mice at 10 weeks of age. In (a), the limb-clasping reflex is observed in Wipi3cKO mice. In (b), the footprint assay indicated a motor deficit in Wipi3cKO mice.
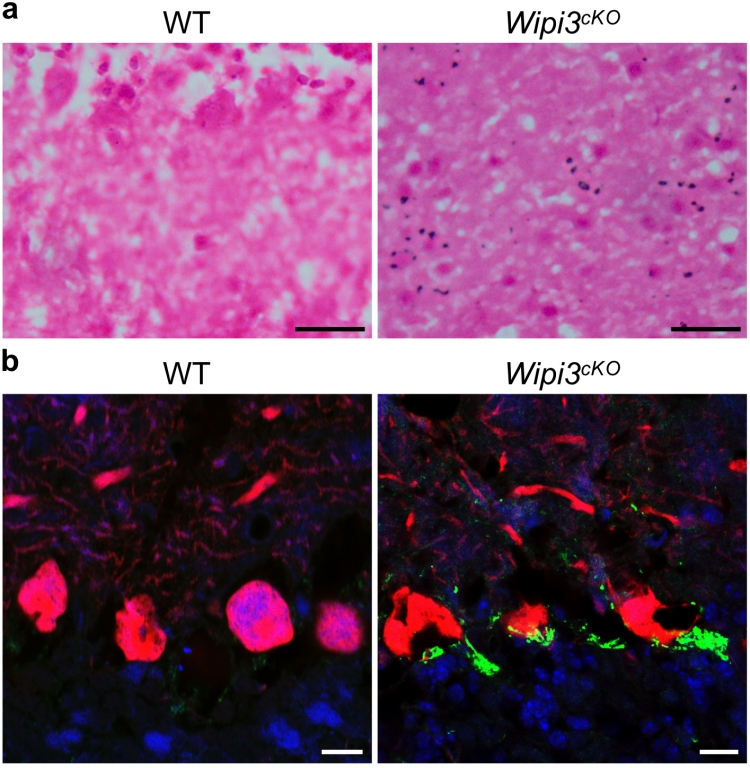
Accumulation of iron and ceruloplasmin in neuron-specific Wipi3cKO mice
Cryosections of the cerebellum from Wipi3cKO mice and WT mice were stained with Prussian blue (a) and were immunostained with anti-ceruloplasmin (green) and anti-calbindin (red) antibodies (b). Blue puncta indicate iron deposition in (a).
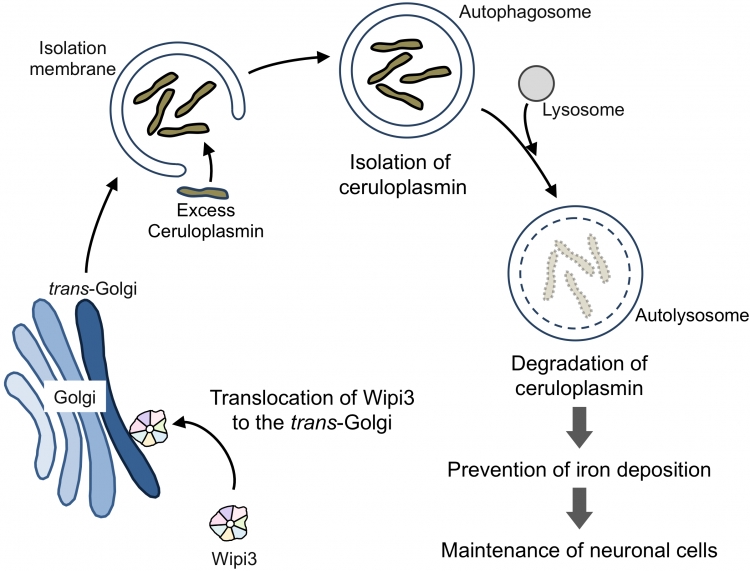
Wipi3 is required for the maintenance of brain cells via alternative autophagy
Wipi3 is translocated from the cytosol to the trans-Golgi, and manipulates the trans-Golgi membrane to generate autophagic vacuoles. In vivo, Wipi3-dependent alternative autophagy degrades excess ceruloplasmin, and prevents abnormal iron deposition in brain cells.
Summary
Correspondence to
Shigeomi SHIMIZU, M.D., Ph.D., Professor
Department of Pathological Cell Biology,
Medical Research Institute,
Tokyo Medical and Dental University(TMDU)
E-mail:arako.pcb(at)mri.tmd.ac.jp
shimizu.pcb(at)mri.tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

