“Breakthrough for Bone Regeneration via Double-cell-layered Tissue Engineering Technique”

Kengo IWASAKI, DDS, PhD, Junior Associate Professor,
Department of Nanomedicine (DNP), Graduate School of Medical and Dental Sciences, TMDU (Right)
Ikuo MORITA, PhD, Executive Director /
Executive Vice President of Research and International Cooperation, TMDU (Center)
Akio KISHIDA, PhD, Professor,
Department of Material-Based Medical Engineering, Institute of Biomaterials and Bioengineering, TMDU (Reft)
Researchers at Tokyo Medical and Dental University (TMDU) develop technique for creating artificial constructs containing two distinct layers of bone-forming cells that can be transplanted onto bone defects, increasing rate of repair
Tokyo, Japan – Various technologies have been developed to introduce laboratory-grown bone-forming cells into bone defects to promote their repair. However, these have many limitations as the conditions of the cells and their surroundings do not accurately mimic those typically found in the body. This means they cannot optimally promote bone formation. A research team at Tokyo Medical and Dental University (TMDU) has now made a major advance in overcoming these difficulties by developing a technique for producing double-layered cell constructs that can be transplanted onto bone defects. The technique increases the speed of bone repair and the flexibility and durability of the constructs make them ideal for many surgical applications.
Cells with various functions can now be cultured in the laboratory and then introduced into the body to treat different medical conditions. However, as individual cells can spread away from the site of injury, they need to be held in place on a scaffold, which is then transplanted into the body. Substantial progress has already been made in this sort of tissue engineering. When the body repairs broken or damaged bones, it employs a complex system of molecular signals and cells, including osteoblasts that build up the calcium matrix on which bone is based. To speed up the repair of bone defects by artificial means or enable recovery from severe injuries, tissue engineering approaches thus need to mimic this complex system.
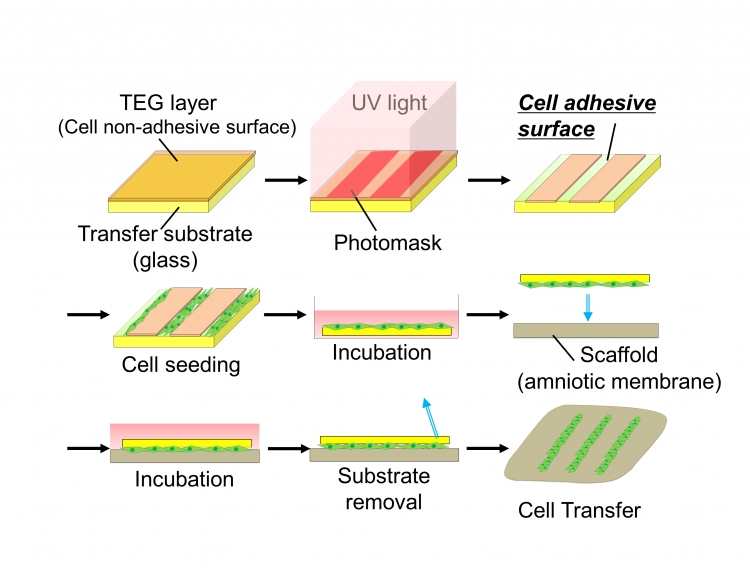
Fig.1 Schematic diagram of cell transfer technology and double-cell transfer
(A) We coated the surface of glass substrate with tetraethylene glycol (TEG, brown) and the layer was partially degraded by UV irradiation to prepare hydrophilic cell adhesive surface (green). Cells to be transferred were poured onto the substrate and incubated to allow the cells to adhere to the substrate surface. Transfer substrate with cells was then placed onto the scaffold (amnion) in the direction of cell surface down. Cells were further cultured and transfer substrate was carefully removed subsequently. Cells are transferred onto scaffold surface.
(B) Cells of the first layer (green) were seeded on the transfer substrate and cultured. Then, the cells of the second layer (red) were seeded onto the cells of the first layer. After incubation, transfer substrate bearing two layers of cells was placed onto the amnion to make direct contact between cells and scaffold surface. Double cell layers were transferred onto the scaffold material after the removal of the transfer base.
“After establishing our double-layered cell transfer technology, we used it to apply different combinations of cells related to bone formation to defects in mouse skulls,” first author Keiko Akazawa says. “We found that osteoblasts together with stem cells from tooth-supporting ligament were particularly effective at promoting bone repair than equivalent scaffolds containing only a single cell layer.”
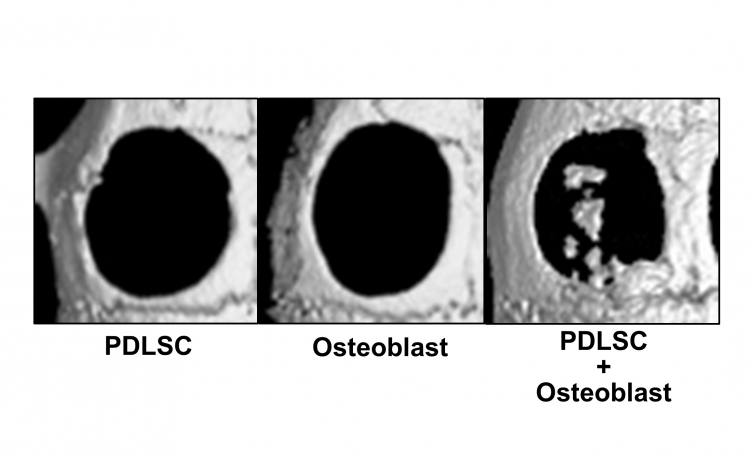
Fig.2 Bone regeneration by cell-transferred amnion
Micro CT images of bone defects 4 weeks after the transplantation of cell-transferred amnion. In the single cell transplantation (mesenchymal stem cells from periodontal ligament (PDLSC) or Osteoblast), bone healing was limited, while new bone-like tissue formation was observed in bone defects transplanted with double-layered cell-transferred amnion (PDLSC+Osteoblast).
The double-layered cell constructs were also tested for their stability and flexibility. The cells remained attached despite folding the constructs or trimming them to fit the shape of a particular defect. Coauthor Kengo Iwasaki says: “The durability of these new constructs makes them particularly suitable for surgical applications. We have high expectations for their use in regenerative medicine for treating a range of defects using different cell layer combinations.”
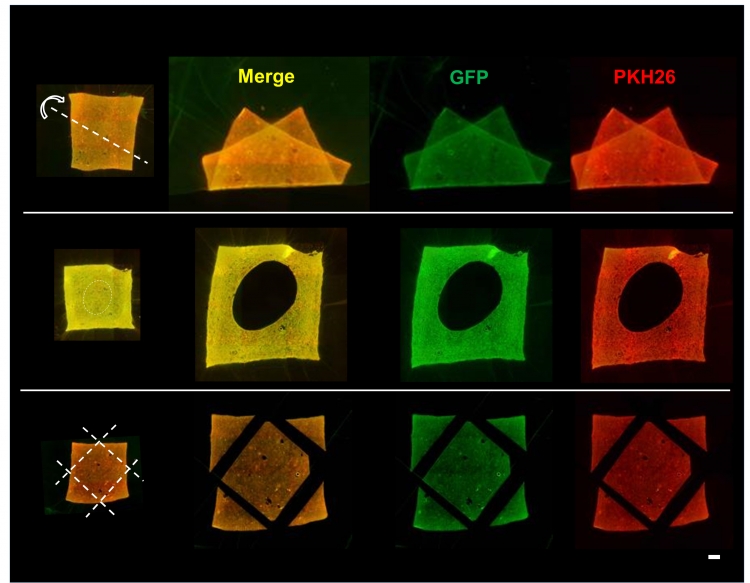
Fig.3 Stability of transferred cells upon trimming and deformation of amnion
Fluorescence microscopic images of amnion holding double-layered cells after deformation (top), holing (middle) and trimming (bottom) of the membrane. Despite of deformations and trimming of cell transferred amnion, cells were stably adhered onto the scaffold material.
Green (GFP): First layer cells, Red (PKH26): Second layer cells. Bar = 1 mm
Summary
Correspondence to:
Department of Nanomedicine (DNP),
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University (TMDU)
E-mail: k-iwasaki.peri(at)tmd.ac.jp

