A New Type of Recyclable: Finding New Uses for Established Drugs
Researchers from Tokyo Medical and Dental University (TMDU) uncover potential novel therapeutic strategies for oral and esophageal carcinomas
Tokyo, Japan – Discovering and treating tumors before they spread throughout the body is key for cancer patients to achieve positive outcomes. When tumor cells spread, which is known as metastasis, they can take over other organs and lead to death. Oral and esophageal carcinomas, or mouth and throat cancers, frequently metastasize to the lymph nodes. Unfortunately, there are currently no therapies that are specific to treating these particular cancers. Now, researchers at Tokyo Medical and Dental University (TMDU) identified several drugs that can possibly be used to treat oral and esophageal carcinomas.
In an article published in Molecular Cancer Research, a group of researchers from TMDU found that combining two drugs, pitavastatin and capmatinib, inhibited the viability of oral cancer cells in culture, as well as the growth of tumors in a mouse model.
Although esophageal carcinoma is the sixth most deadly cancer worldwide and is relatively well understood at the molecular level, the research has not been translated into specific therapeutic development. Because of this urgent need, the TMDU group became interested in drug repurposing, where a drug that has been approved for a certain disease can be used to effectively treat an additional indication. This concept significantly speeds up the drug discovery and development process, increasing the number of patients that can benefit from an established therapeutic.
“Drug repurposing can be extremely helpful for discovering efficacious treatments for diseases lacking approved therapies,” says lead author of the study Tomoki Muramatsu “We began this process for oral and esophageal carcinomas by screening an FDA-approved drug library.”
The researchers performed the drug screening on a highly metastatic oral cancer cell line. Overall, the drug pitavastatin reduced the growth of these cells most significantly. Through molecular analysis, they determined that pitavastatin acted by inhibiting a cellular pathway called MET signaling. Because of this, the researchers added in a second MET inhibitor drug, known as capmatinib.
“Combining pitavastatin with capmatinib resulted in an even greater reduction in cancer cell growth,” describes senior author Johji Inazawa. “Capmatinib by itself had no effect on the cancer cells, but it synergized with pitavastatin.”
The researchers then injected these cells into mice to generate tumors and observed a similar effect with the pitavastatin and capmatinib combination.
“Our results in the mouse model corroborated our in vitro findings,” says Muramatsu. “The data suggest that MET signaling may be a valuable therapeutic target in these tumors.”
The study also identified a potential biomarker for these particular cancers – a gene called GGPS1. Expression levels of this gene may correlate with patient responsiveness to pitavastatin. This work provides knowledge that may be vital for identifying therapeutics for these devastating diseases.
Tokyo, Japan – Discovering and treating tumors before they spread throughout the body is key for cancer patients to achieve positive outcomes. When tumor cells spread, which is known as metastasis, they can take over other organs and lead to death. Oral and esophageal carcinomas, or mouth and throat cancers, frequently metastasize to the lymph nodes. Unfortunately, there are currently no therapies that are specific to treating these particular cancers. Now, researchers at Tokyo Medical and Dental University (TMDU) identified several drugs that can possibly be used to treat oral and esophageal carcinomas.
In an article published in Molecular Cancer Research, a group of researchers from TMDU found that combining two drugs, pitavastatin and capmatinib, inhibited the viability of oral cancer cells in culture, as well as the growth of tumors in a mouse model.
Although esophageal carcinoma is the sixth most deadly cancer worldwide and is relatively well understood at the molecular level, the research has not been translated into specific therapeutic development. Because of this urgent need, the TMDU group became interested in drug repurposing, where a drug that has been approved for a certain disease can be used to effectively treat an additional indication. This concept significantly speeds up the drug discovery and development process, increasing the number of patients that can benefit from an established therapeutic.
“Drug repurposing can be extremely helpful for discovering efficacious treatments for diseases lacking approved therapies,” says lead author of the study Tomoki Muramatsu “We began this process for oral and esophageal carcinomas by screening an FDA-approved drug library.”
The researchers performed the drug screening on a highly metastatic oral cancer cell line. Overall, the drug pitavastatin reduced the growth of these cells most significantly. Through molecular analysis, they determined that pitavastatin acted by inhibiting a cellular pathway called MET signaling. Because of this, the researchers added in a second MET inhibitor drug, known as capmatinib.
“Combining pitavastatin with capmatinib resulted in an even greater reduction in cancer cell growth,” describes senior author Johji Inazawa. “Capmatinib by itself had no effect on the cancer cells, but it synergized with pitavastatin.”
The researchers then injected these cells into mice to generate tumors and observed a similar effect with the pitavastatin and capmatinib combination.
“Our results in the mouse model corroborated our in vitro findings,” says Muramatsu. “The data suggest that MET signaling may be a valuable therapeutic target in these tumors.”
The study also identified a potential biomarker for these particular cancers – a gene called GGPS1. Expression levels of this gene may correlate with patient responsiveness to pitavastatin. This work provides knowledge that may be vital for identifying therapeutics for these devastating diseases.
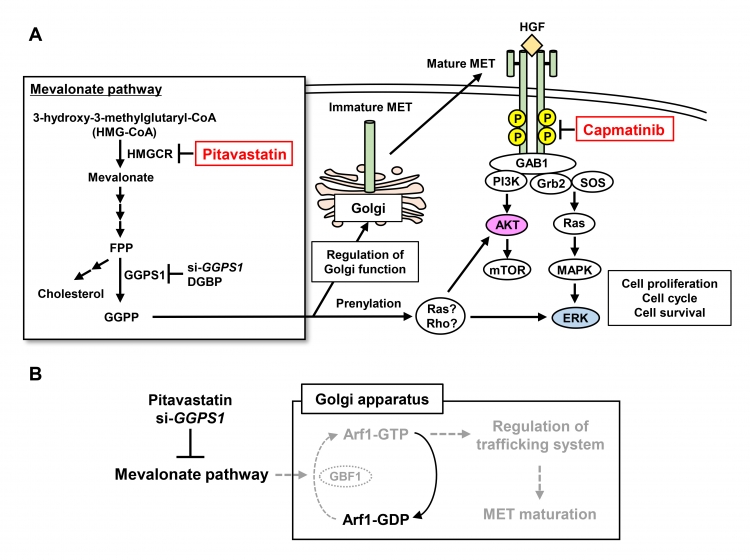
The anticancer functions of the combination of pitavastatin and capmatinib
A, The relationship between the mevalonate pathway and each survival or growth pathway.
B, Induction of dysfunction of the Golgi apparatus by inhibition of the mevalonate pathway.
###
The article, “Suppression of MET signaling mediated by pitavastatin and capmatinib inhibits oral and esophageal cancer cell growth,” was published in Molecular Cancer Research at DOI: 10.1158/1541-7786.MCR-20-0688.
The article, “Suppression of MET signaling mediated by pitavastatin and capmatinib inhibits oral and esophageal cancer cell growth,” was published in Molecular Cancer Research at DOI: 10.1158/1541-7786.MCR-20-0688.
Summary
Using cell culture and mouse model approaches, researchers from Tokyo Medical and Dental University (TMDU) have found a novel drug combination that can inhibit tumor growth in oral and esophageal carcinomas. They also identified a potential biomarker that can help predict patient sensitivity to this treatment. These findings provide valuable knowledge for the fight against these deadly cancers and demonstrate a potential new use for drugs currently approved for other diseases.
Correspondence to
Tomoki Muramatsu, Ph.D., Assistant Professor
Johji Inazawa, M.D., Ph.D., Professor
Department of Molecular Cytogenetics,
Medical Research Institute,
Tokyo Medical and Dental University(TMDU)
E-mail:johinaz.cgen (at) mri.tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.
Johji Inazawa, M.D., Ph.D., Professor
Department of Molecular Cytogenetics,
Medical Research Institute,
Tokyo Medical and Dental University(TMDU)
E-mail:johinaz.cgen (at) mri.tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

