“Identification of a novel mechanism by which allergic inflammation is dampened”

Hajime KARASUYAMA
Professor
Department of Immune Regulation,
Graduate School of Medical and Dental Sciences (right)
Mayumi EGAWA
Graduate Student
Department of Immune Regulation,
Graduate School of Medical and Dental Sciences (left)
Highlights
| 1. | As many as ~30% of people in Japan suffer from allergic disorders including allergic rhinitis, atopic dermatitis, and asthma. |
| 2. | The mechanism for the development of allergy has been extensively studied while that for the suppression and termination of allergy has been poorly understood. |
| 3. | The present study identified a novel mechanism by which allergic inflammation is dampened, via conversion of inflammation-inducing (proinflammatory) cells to inflammation-suppressing (anti-inflammatory) cells. |
| 4. | This finding would promote the development of a novel strategy for the treatment of allergic disorders. |
Main findings and significance of this study
Figures
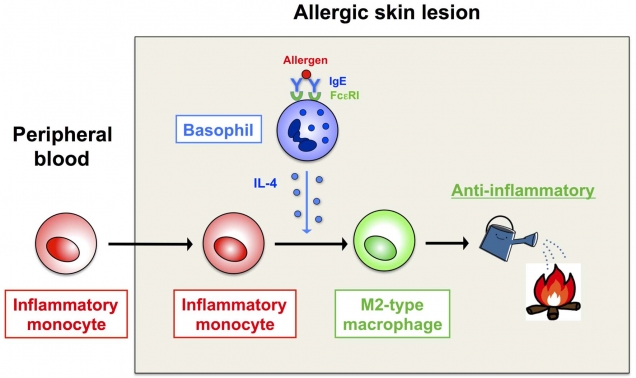
Figure 1. Conversion of immune cells from being proinflammatory (inflammatory monocytes) to anti-inflammatory (type 2 macrophages)
Inflammatory monocytes recruited to the allergen-exposed skin differentiate into type 2 macrophages, under the influence of a cytokine (interleukin-4) produced by basophils, and dampen allergic inflammation.
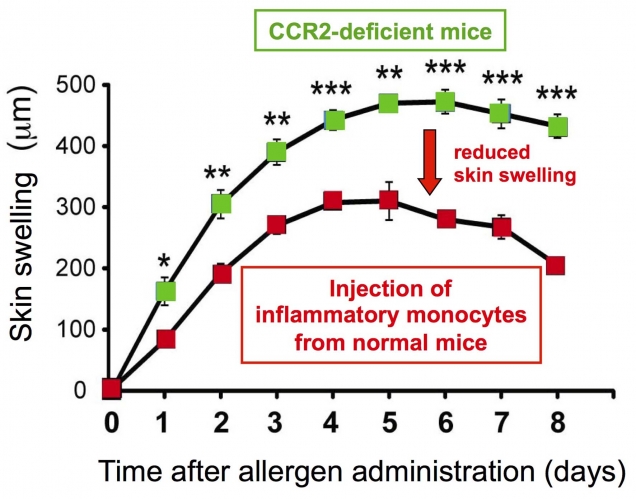
Figure 2. Inflammatory monocytes show anti-inflammatory property after their migration to allergic skin
Mice lacking a chemokine receptor CCR2 show severer and prolonged skin swelling due to impaired migration of inflammatory monocytes to the skin lesions. Injection of CCR2-expressing inflammatory monocytes isolated from normal mice into CCR2-deficient mice dampens the exacerbated allergic inflammation in the skin of CCR2-deficient mice.
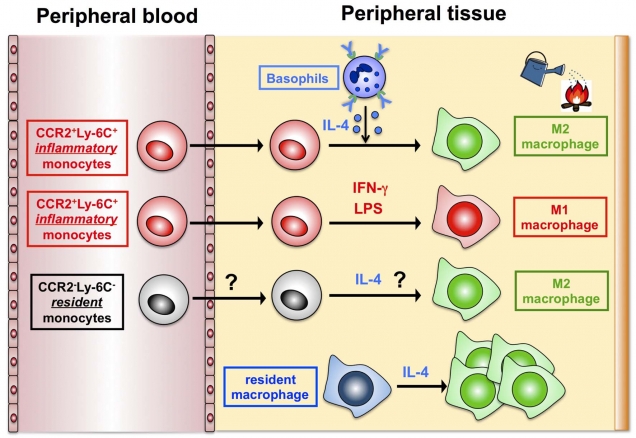
Figure 3. A novel mode of type 2 macrophage generation
It is well known that blood-circulating, inflammatory monocytes differentiate into type 1 macrophages that show an inflammatory property, after their recruitment to peripheral tissues. In contrast, the mode of type 2 macrophage generation has been poorly understood. The present study identified a novel mode of type 2 macrophage generation, that is, the differentiation from blood-circulating, inflammatory monocytes, besides previously-reported modes: the generation from blood-circulating, resident monocytes and that from tissue-resident macrophages.
Correspondence to:
Department of Immune Regulation
Tokyo Medical and Dental University, Graduate School of Medical and Dental Sciences
1-5-45 Yushima, Bunkyo-ku, Tokyo, Japan 113-8519
Phone: +81-3-5803-5162
E-mail: karasuyama.mbch(at)tmd.ac.jp
URL: http://immune-regulation.org/index.php?id=27
*Please change (at) in e-mail addresses to @ upon sending your e-mail to the contact personnel.

