“Development of non-human primate model of amyotrophic lateral sclerosis (ALS)”

Takanori Yokota
Professor,
Department of Neurology and Neurological Science,
Graduate School of Medical and Dental Sciences (right)
Hidehiro Mizusawa
Chairman,
Department of Neurology and Neurological Science,
Graduate School of Medical and Dental Sciences (left)
Point
| ● | We first developed a non-human primate model of ALS with cytoplasmic mislocalization of TDP-43. |
| ● | There is a species difference in TDP-43 pathology, and our monkey model recapitulates ALS pathology to a much greater extent than rodent models, providing a valuable tool for studying the pathogenesis and developing therapy of sporadic ALS. |
Content of the research
Contact
Department of Neurology and Neurological Science,
Tokyo Medical and Dental University
1-5-45 Yushima Bunkyo-ku, Toko 113-8519
Phone: +81-3-5803-5234
FAX: +81-3-5803-0169
email: tak-yokota.nuro(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.
Figure
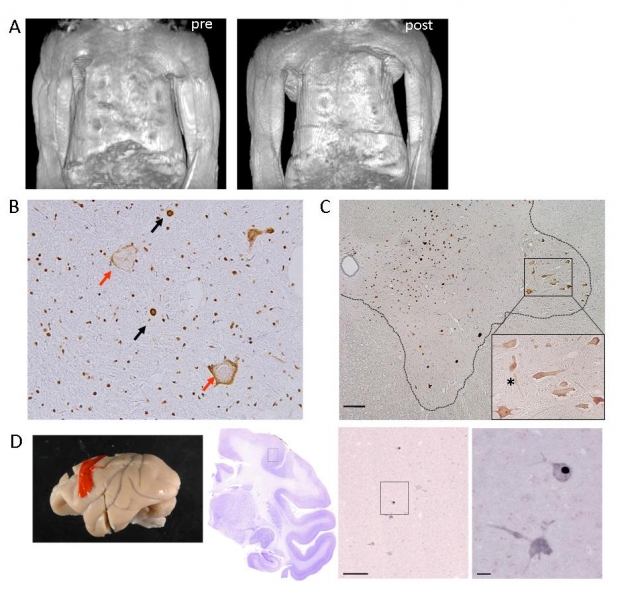
Overexpressing wild-type TDP-43 in the monkey cervical cord leads to progressive motor weakness and muscle atrophy. Motor paresis of the forelimb on the injected side (encircled) in TDP-43 AAV-injected monkeys. (A) Constructed MRI images of upper arm muscles before (pre) and 4 weeks after (post) injection, indicating a marked muscle atrophy of upper and forearms as well as hand muscles, predominantly on the injected side (left side). (B) Immunostaining of the cervical cord sections of TDP-43–injected monkeys at the late stage using antibodies to pan-TDP-43 demonstrated normal localization in nuclei (black arrows), mislocalization in cytoplasm (red arrows). Regional mislocalization of TDP-43 and cell death in monkey spinal cords. (C) Sections from the injected side of the 8th cervical segment of the cord taken at the early stage and immunostained with an anti-Flag antibody. Most neurons in the lateral nuclear group (area encircled by broken line) showed cytoplasmic mislocalization of TDP-43 (enlarged inset), but almost all neurons in other areas expressed exogenous TDP-43 in the nucleus.(D) Immunostaining with anti-Flag antibody indicated that exogenous flag TDP-43 is expressed in nucleus or cytoplasm of the Betz cells in the primary motor cortex. Scale bars: 200 μm.

