From blood to brain: delivering nucleic acid therapy to the CNS

Evolution has equipped the brain with protection against both mechanical and molecular injury. The blood–brain barrier (BBB) is a selectively semipermeable barricade of endothelial cells lining the capillaries; working with specific transporter proteins, it functions as a fastidious gatekeeper between the circulation and the CNS, barring foreign molecules, including drugs.
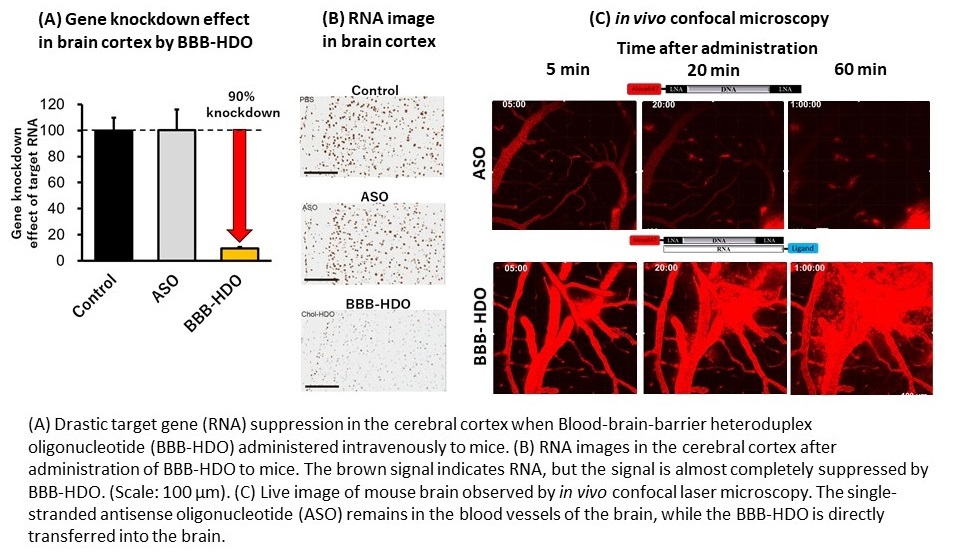
The research team had recently developed DNA/RNA heteroduplex oligonucleotide (HDO) technology capable of highly efficient RNA degradation in vivo. First author Tetsuya Nagata explains, “We found that cholesterol conjugated HDO (Chol-HDO), unlike cholesterol-ASO, efficiently reached the CNS following subcutaneous or intravenous administration in experimental animals. The Chol-HDO platform showed significant dose-dependent target gene reductions with prolonged action in all CNS regions and cell types.”
“Systemic doses being higher, adverse effects such as mild decrease in platelets were expected,” says Nagata. “However, divided or subcutaneous dosing can rescue these. We may also strategize by initiating treatment with intrathecal dosing to rapidly achieve therapeutic concentrations, followed by intravenous or subcutaneous maintenance as needed.”
Our innovative therapeutic platform for blood-to-brain delivery of ASOs may revolutionize management of neurodegenerative diseases,” senior author Takanori Yokota claims. “Future research will help define the specific molecular pathways thus optimizing delivery of ASO pharmacotherapy to the CNS.”
Paper Information
Tetsuya Nagata, Chrissa A. Dwyer, Kie Tanaka-Yoshida, Kensuke Ihara, Masaki Ohyagi, Haruka Miyata, Satoe Ebihara, Kotaro Yoshioka, Takashi Ishii, Kanjiro Miyata, Kenichi Miyata, Berit Powers, Tomoko Igari, Syunsuke Yamamoto, Naoto Arimura, Hideki Hirabayashi, Toshiki Uchihara, Rintaro Iwata Hara, Takeshi Wada, C. Frank Bennett, Punit P. Seth, Frank Rigo and Takanori Yokota
Publication: Nature Biotechnology
Publication Date: 2021 Aug 12. Online ahead of print. DOI: 10.1038/s41587-021-00972-x
Correspondence to
Department of Neurology and Neurological Science,
Graduate School of Medical and Dental Sciences,
Tokyo Medical and Dental University(TMDU)
E-mail: tak-yokota.nuro(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

