Self-organization of vascularized organoids allows enhanced survival of islet transplants for use in diabetes therapy
PDF Download
- Vascularized organoids for diabetes therapy

Takanori Takebe
Professor of Organogenesis Unit at TMDU
Dr. Takebe is a Professor at TMDU and Associate Director of the Center for Stem Cell and Organoid Medicine (CuSTOM) at the Cincinnati Children's Hospital Medical Center. He serves on the Board of Directors at the International Society of Stem Cell Research (ISSCR) and has received numerous awards, including the NYSCF Robertson Investigator Award. His lab investigates the mechanisms of human organogenesis, and develops mini-organ technologies from human stem cells - namely organ bud-based approaches. He is applying iPSC-liver buds into drug discovery studies as well as transplant applications for patients with a rare congenital metabolic disorder. His work will ultimately expand the clinical applications to diseases like liver cirrhosis.
A: Tissue-based therapies are hailed as the next-generation treatment for organ dysfunction. They generally involve the harvesting of tissues, rather than whole organs, from donors. Much of the enthusiasm for this research has been driven by the partial success of pancreatic islet transplantation,which was initially described nearly 20 years ago. A related approach, stem cell-based tissue engineering, aims to provide transplantable tissue without requiring an organ donor. However, both approaches are limited by a lack of success in ensuring efficient formation of new blood vessels, a process known as vascularization. The timely establishment of tissue vascularization within engineered tissue is necessary to ensure its survival and proper functionality in vitro, as well as to achieve successful tissue engraftment within the patient’s body, followed by appropriate performance of its expected function in vivo.

A: A well-known example of clinical transplantation of tissue fragments involves pancreatic islet transplantation, which promotes insulin independence in patients with severe type 1 diabetes. An important limitation of this method is that transplanted islets have a disappointingly low engraftment rate because these islets lose vasculature during the isolation process. This lack of vasculature induces necrosis, reducing the treatment efficacy. Therefore, rapid establishment of vascular networks is critical for successful engraftment of transplanted islets. Thus far, transplant vascularization generally requires at least a week: rapidly introducing vasculature into transplanted tissue remains challenging
A: Recently, we developed a dynamic self-condensation approach to develop tissue organoids from dissociated organ progenitor cells (early descendants of stem cells that retain the ability to become a broad, but not unlimited, variety of cell types), together with stromal vascular and mesenchymal progenitors. Although we achieved rapid blood vessel induction in tissue organoids generated from single, dissociated cells in suspension, it was not clear whether tissue fragments, such as islets, could also be adapted to follow this self-condensation principle. In the current publication, we showed multiple types of tissue fragments can self-organize three-dimensional tissue structures with developing vascular networks by following the self-condensation culture approach. This method not merely enables an increased scale for self-condensation, but allows for integrating supportive lineages, such as endothelial lineages. Moreover, this method uses primary pancreatic tissues, which are preferable to MIN6 cells used in prior studies that were isolated from an insulinoma of a transgenic mouse expressing the SV40 T antigen in pancreatic islet beta cells. Given that recently evolving organoid-based approaches generally omit vasculature, our methodology can support building additional complexity into engineered tissues or organoids. By enabling the construction of complex and heterotypic structures, this self-condensation principle will aid in disease modeling and drug discovery, and ultimately in regenerative medicine applications.
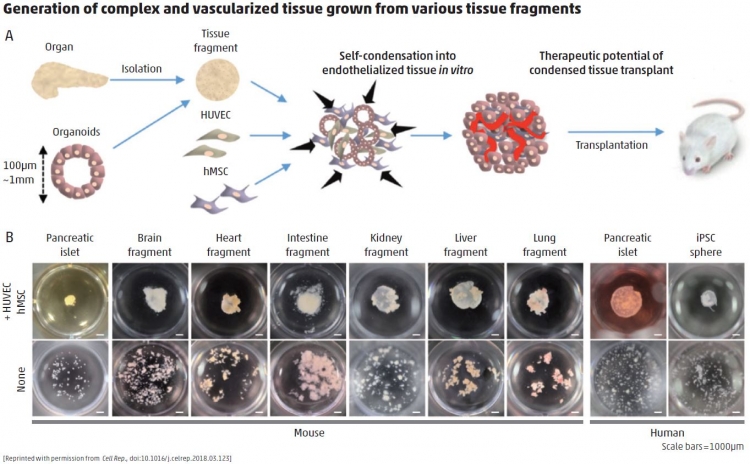
A: The primary challenges of islet transplantation are treating patients using a minimal number of donors and achieving stable and long-term glycemic control after transplantation. In diabetic mice, the approach that my colleagues and I have tested showed dramatic improvement of survival rates. These findings were supported by improved islet engraftment rates, insulin secretion function, and glucose responsiveness. One day, we expect that transplantation of vascularized islets into patients with type 1 diabetes may promote long-term insulin independence. Of note, this method might reduce the number of donors required to treat a single patient, which would increase the capacity of the medical community to manage this disease. We hope that success in the treatment of patients with diabetes might entice researchers in other fields to use this approach with complex tissues involved in other diseases, so that we can deliver curative therapies to a broad number of patients with a variety of genetic and lifestyle or environmental pathologies.

