“My Medicine” with organoids– Predicting the future of liver-safe drugs

The ancient Romans studied the livers of sacrificial animals to read omens and make prophesies. Now researchers at Tokyo Medical and Dental University (TMDU) and Takeda-CiRA program along with a world-wide team of collaborators, have devised a polygenic risk score (PRS) based on liver genomics that can predict the likelihood of medications causing liver damage.
Introducing new drugs is a demanding process. Pharmaceutical research continually proffers potential drugs that need to be clinically trialed. These candidates are often more efficacious, but may have unacceptable or unsuspected side-effects. Unfortunately, adverse outcomes often require termination of new drug trials, and even drugs in common use may show a cumulated trend of undesired effects hitherto unpredicted; identifying patients at risk can greatly reduce this.
Introducing new drugs is a demanding process. Pharmaceutical research continually proffers potential drugs that need to be clinically trialed. These candidates are often more efficacious, but may have unacceptable or unsuspected side-effects. Unfortunately, adverse outcomes often require termination of new drug trials, and even drugs in common use may show a cumulated trend of undesired effects hitherto unpredicted; identifying patients at risk can greatly reduce this.
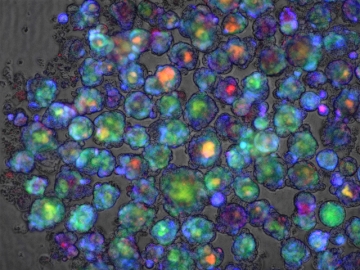
Color-stained confocal microscope image of multiple liver organoids used in this study
Green: Bile acid, Red: PI, Blue: ROS in human liver organoids
The liver is the primary site where most drugs, indeed any foreign potentially toxic chemical, is metabolized into an inactive form for excretion by the body. As a “frontliner”, it bears the brunt of most adverse effects that manifest as hepatocyte injury. Indeed, drug-induced-liver-injury (DILI) is the main reason why drugs are withdrawn at different stages of development, trial and usage, often after significant, and avoidable, morbidity and expense.
“We formulated our risk score by mathematically analyzing previous genome-wide association studies that had flagged variants likely to predict susceptibility to DILI,” explains Masaru Koido, lead author. “We validated it across a spectrum of potentially hepatotoxic drugs, on genomic data, primary hepatocyte cultures and organoids from multiple donors. Noteworthy was our use of organoids—mini-organs bioengineered from three-dimensional tissue cultures derived from stem cells that replicate their microanatomy and functional complexity.”
“We formulated our risk score by mathematically analyzing previous genome-wide association studies that had flagged variants likely to predict susceptibility to DILI,” explains Masaru Koido, lead author. “We validated it across a spectrum of potentially hepatotoxic drugs, on genomic data, primary hepatocyte cultures and organoids from multiple donors. Noteworthy was our use of organoids—mini-organs bioengineered from three-dimensional tissue cultures derived from stem cells that replicate their microanatomy and functional complexity.”

Illustration of "polygenicity in a dish" strategy
Genotyped human liver organoids will be tested to determine patients’ precision against drug induced effects.
The researchers also analyzed the derived scores to delineate pathways underlying susceptibility to DILI. From the data they inferred that genetic variation at the hepatocyte level contributed to DILI susceptibility; moreover, DILI predictivity was shared across a variety of discrete drugs suggesting that the PRS related to intracellular mechanisms of hepatotoxicity.
“Our “polygenicity-in-a-dish” strategy allows safe, specific and multidimensional investigation into the pathogenesis of DILI,” explains senior author Takanori Takebe. “A genetic test score will enable clinicians to tailor medication choice, dosage, and monitoring based on the patient’s estimated risk. Furthermore, drug trials could be made safer and better focused by excluding vulnerable subjects. However, further research is needed to upscale our PRS into a valid and reliable instrument for widespread screening of novel pharmaceuticals in clinical practice.
“Our “polygenicity-in-a-dish” strategy allows safe, specific and multidimensional investigation into the pathogenesis of DILI,” explains senior author Takanori Takebe. “A genetic test score will enable clinicians to tailor medication choice, dosage, and monitoring based on the patient’s estimated risk. Furthermore, drug trials could be made safer and better focused by excluding vulnerable subjects. However, further research is needed to upscale our PRS into a valid and reliable instrument for widespread screening of novel pharmaceuticals in clinical practice.
Paper Information
Polygenic architecture informs potential vulnerability to drug-induced liver injury
Masaru Koido, Eri Kawakami, Junko Fukumura, Yui Noguchi, Momoko Ohori, Yasunori Nio, Paola Nicoletti, Guruprasad P Aithal, Ann K Daly, Paul B Watkins, Hisashi Anayama, Yvonne Dragan, Tadahiro Shinozawa, Takanori Takebe
Publication: Nature Medicine, 2020 Oct;26(10):1541-1548.
Publication Date: October 2020 DOI: 10.1038/s41591-020-1023-0
Masaru Koido, Eri Kawakami, Junko Fukumura, Yui Noguchi, Momoko Ohori, Yasunori Nio, Paola Nicoletti, Guruprasad P Aithal, Ann K Daly, Paul B Watkins, Hisashi Anayama, Yvonne Dragan, Tadahiro Shinozawa, Takanori Takebe
Publication: Nature Medicine, 2020 Oct;26(10):1541-1548.
Publication Date: October 2020 DOI: 10.1038/s41591-020-1023-0
Correspondence to
Takanori TAKEBE, Professor
Institute of Research, Division of Advanced Research
Tokyo Medical and Dental University(TMDU)
E-mail:ttakebe.ior(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.
Institute of Research, Division of Advanced Research
Tokyo Medical and Dental University(TMDU)
E-mail:ttakebe.ior(at)tmd.ac.jp
*Please change (at) in e-mail addresses to @ on sending your e-mail to contact personnels.

