Therapeutic targets in chronic active EBV infection

Mayumi Yoshimori,
Specially Appointed Assistant Professor of Laboratory Molecular Genetics of Hematology at TMDU
After graduating from TMDU, I worked as a medical technologist for five years. In 2017, I decided to become a medical researcher and started my career in my present position. My research subject has been clarifying the molecular mechanisms of the development of chronic active Epstein-Barr virus infection (CAEBV).
EBV is a common virus. Once EBV infects human beings, it cannot be eradicated and latently infects B cells throughout the lifespan. However, EBV genome is also positive in some T- or NK-cell neoplasms. CAEBV is one of them with a poor prognosis. The mechanisms of the development of CAEBV have not been clarified and the only current curative strategy is hematopoietic
stem cell transplantation. CAEBV has a marked geographic bias for East Asia, suggesting a genetic context for disease development. However, CAEBV certainly exists in Western countries, too. As Japanese researchers, we feel responsible for addressing and elucidating the issues of CAEBV.
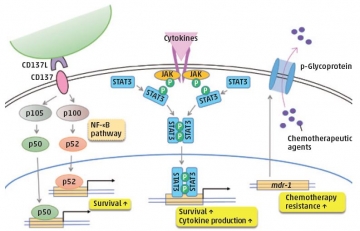
During the graduate course, we demonstrated that in vitro infection of EBV in T cells upregulated the CD137 expression and promoted the survival of infected cells (PLoS One, doi: 10.1371/journal.pone.0112564). We also found that EBV infection of T cells enhanced P-glycoprotein expression in the infected cells, contributing to CAEBV’s resistance to chemotherapy (Cancer Med., doi: 10.1002/cam4.494 ). Our recent studies have revealed that constitutively activated STAT3 promoted cell survival and cytokine production, which had been suppressed by the effect of ruxolitinib (Oncotarget, doi:10.18632/oncotarget.25780). Based on these findings, we are now planning to initiate clinical trials of ruxolitinib for CAEBV.