Targeting intercellular communication between bone cells

Mikihito Hayashi,
Assistant Professor of Cell Signaling at TMDU
Bone is a constantly renewing organ throughout life. This is characterized predominantly by skeletal growth during development and childhood, and a subsequent continuous turnover, known as “bone remodeling”. It is a finely balanced activity and carried out by specialized groups of cells, including osteoclasts, which are multinucleated cells that resorb bone, and osteoblasts, which refill the resorption cavities created by osteoclasts. An imbalance between bone resorption and formation results in metabolic bone disorders such as osteoporosis, a disease of low bone mass with increased susceptibility to bone fractures.
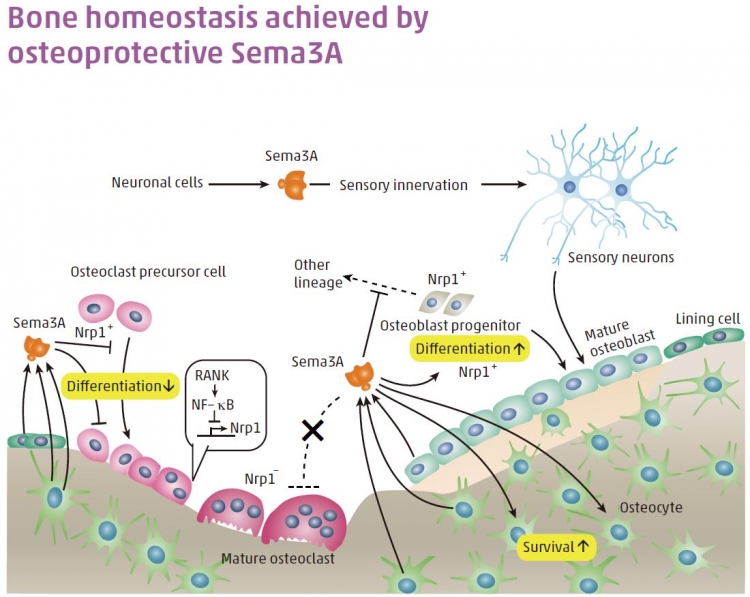
We demonstrated that the essential source of RANKL, a critical factor for osteoclast diff erentiation, is osteocytes, which are terminally diff erentiated osteoblasts embedded in bone matrix (Nat. Med., doi:10.1038/nm.2452). We also demonstrated that Sema3A, which was originally identifi ed as a guidance factor for developing axons, plays an important role in the regulation
of cell-to-cell communication between osteoclasts, osteoblasts and osteocytes during bone remodeling (Figure; Nature, doi:10.1038/ nature11000). We are now trying to demonstrate that osteocyte-derived Sema3A, which is under the control of estrogen, acts on osteocyte survival, along with the inhibition of osteoclastic bone resorption and the activation of osteoblastic bone formation.
After completing my PhD at TMDU in 2010, I worked as a postdoctoral fellow of JST(Japan Science and Technology Agency) from 2010 to 2012. In 2012, I became an Assistant Professor at TMDU. The goal of our laboratory’s research is to identify the communication factors involved in bone cells and other tissues and organs, in addition to understanding the osteocyte-mediated response to mechanical stress.