PQBP5/NOL10 maintains and anchors the nucleolus under physiological and osmotic stress conditions (2023)
In a study published recently in Nature Communications, we have revealed that an intrinsically disordered protein (IDP), named "PQBP5", is crucial for the stability of an organelle called the nucleolus.
The nucleolus is critical for transcribing ribosomal DNA, which encodes crucial components of the ribosome, an essential organelle for cellular maintenance, differentiation, and stress responses. Many proteins that are part of the nucleolus are IDPs that are susceptible to deformation and dysfunction in response to stressors such as temperature changes, low oxygen conditions, or dehydration.
We previously identified the IDP polyglutamine binding protein 5 (PQBP5), also known as nucleolar protein 10 (NOL10), in a screen for proteins that bind to polyglutamine (polyQ) tract sequences in proteins that cause polyQ diseases. PQBP5/NOL10 was later found to be a component of the nucleolus. the integrity of which has been implicated in the pathophysiology of neurodegenerative diseases.
To determine whether PQBP5 is involved in maintaining the structural integrity of the nucleolus, we investigated its molecular characteristics, nucleolar sublocalization, relationship with other nucleolar proteins, and stress responses.
Unexpectedly, we found that PQBP5 is a core structural element of the nucleolus, forming a meshwork that supports other nucleolar substructures. Even more intriguingly, unlike other nucleolar proteins that disperse to the nucleoplasm under osmotic stress conditions, PQBP5 remains in the nucleolus and anchors reassembly of the nucleolar structure.
In addition, we found that PQBP5 can essentially be sponged up by polyQ disease proteins, both in cells and in mice. This leads to deformation or even disappearance of the nucleoli. Taken together, these findings indicate that PQBP5 is an essential protein needed to maintain the structure of the nucleolus.
Given that polyQ proteins form aggregates with a dense core that often excludes polyQ-binding proteins, PQBP5 may initially interact with soluble forms of these proteins before being pulled into larger inclusions. Aggregation inhibitors that prevent inclusion formation could therefore affect PQBP5 distribution, and thus nucleolar stability, providing a novel approach to treating polyQ diseases.
The nucleolus is critical for transcribing ribosomal DNA, which encodes crucial components of the ribosome, an essential organelle for cellular maintenance, differentiation, and stress responses. Many proteins that are part of the nucleolus are IDPs that are susceptible to deformation and dysfunction in response to stressors such as temperature changes, low oxygen conditions, or dehydration.
We previously identified the IDP polyglutamine binding protein 5 (PQBP5), also known as nucleolar protein 10 (NOL10), in a screen for proteins that bind to polyglutamine (polyQ) tract sequences in proteins that cause polyQ diseases. PQBP5/NOL10 was later found to be a component of the nucleolus. the integrity of which has been implicated in the pathophysiology of neurodegenerative diseases.
To determine whether PQBP5 is involved in maintaining the structural integrity of the nucleolus, we investigated its molecular characteristics, nucleolar sublocalization, relationship with other nucleolar proteins, and stress responses.
Unexpectedly, we found that PQBP5 is a core structural element of the nucleolus, forming a meshwork that supports other nucleolar substructures. Even more intriguingly, unlike other nucleolar proteins that disperse to the nucleoplasm under osmotic stress conditions, PQBP5 remains in the nucleolus and anchors reassembly of the nucleolar structure.
In addition, we found that PQBP5 can essentially be sponged up by polyQ disease proteins, both in cells and in mice. This leads to deformation or even disappearance of the nucleoli. Taken together, these findings indicate that PQBP5 is an essential protein needed to maintain the structure of the nucleolus.
Given that polyQ proteins form aggregates with a dense core that often excludes polyQ-binding proteins, PQBP5 may initially interact with soluble forms of these proteins before being pulled into larger inclusions. Aggregation inhibitors that prevent inclusion formation could therefore affect PQBP5 distribution, and thus nucleolar stability, providing a novel approach to treating polyQ diseases.
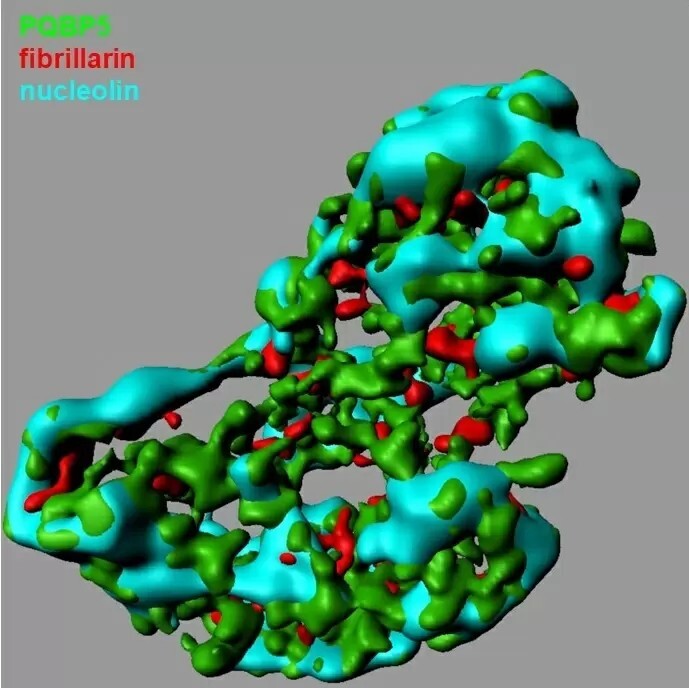
Figure 1 (or Video 1): PQBP5 is an essential structural component of the nucleolus. This 3D image of a single nucleolus shows the interactions among three nucleolar proteins: PQBP5 (green) forms a lattice-like meshwork ball that anchors assembly of fibrillarin (red) and nucleolin (blue).
Publications
Jin, X., Tanaka, H., Jin, M., Fujita, K., Homma, H., Inotsume, M., Yong, H., Umeda, K., Kodera, N., Ando, T. & Okazawa, H. (2023)
PQBP5/NOL10 maintains and anchors the nucleolus under physiological and osmotic stress conditions.
Nat Commun 2023, 14 (1), 9. doi: 10.1038/s41467-022-35602-w
PQBP5/NOL10 maintains and anchors the nucleolus under physiological and osmotic stress conditions.
Nat Commun 2023, 14 (1), 9. doi: 10.1038/s41467-022-35602-w

