Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation (2021)
We have revealed the role of intracellular receptor PQBP1 in response to Tau, a proteinfound primarily in neurons that plays a key role in the progression of neurodegenerative disorders like Alzheimer's disease.
PQBP1 has been previously shown to sense and bind HIV DNA and trigger an immune pathway known as the cGAS-STING pathway to initiate an inflammatory response. While PQBP1 also been shown to interact with dysfunctional proteins such as those implicated in the neruodegenerative disorder Huntington's disease, the specific role of PQBP1 in neurodegenerative inflammatory responses was unclear. To better understand this relationship, researchers from TMDU set out to clarify the nature of the interaction between PQBP1 and Tau.
By characterizing the relationship between PQBP1 and Tau, we were able to clarify a mechanism of inflammation in teh brain that functions in both viral infection and neurodegenerative disease (Figure 1).
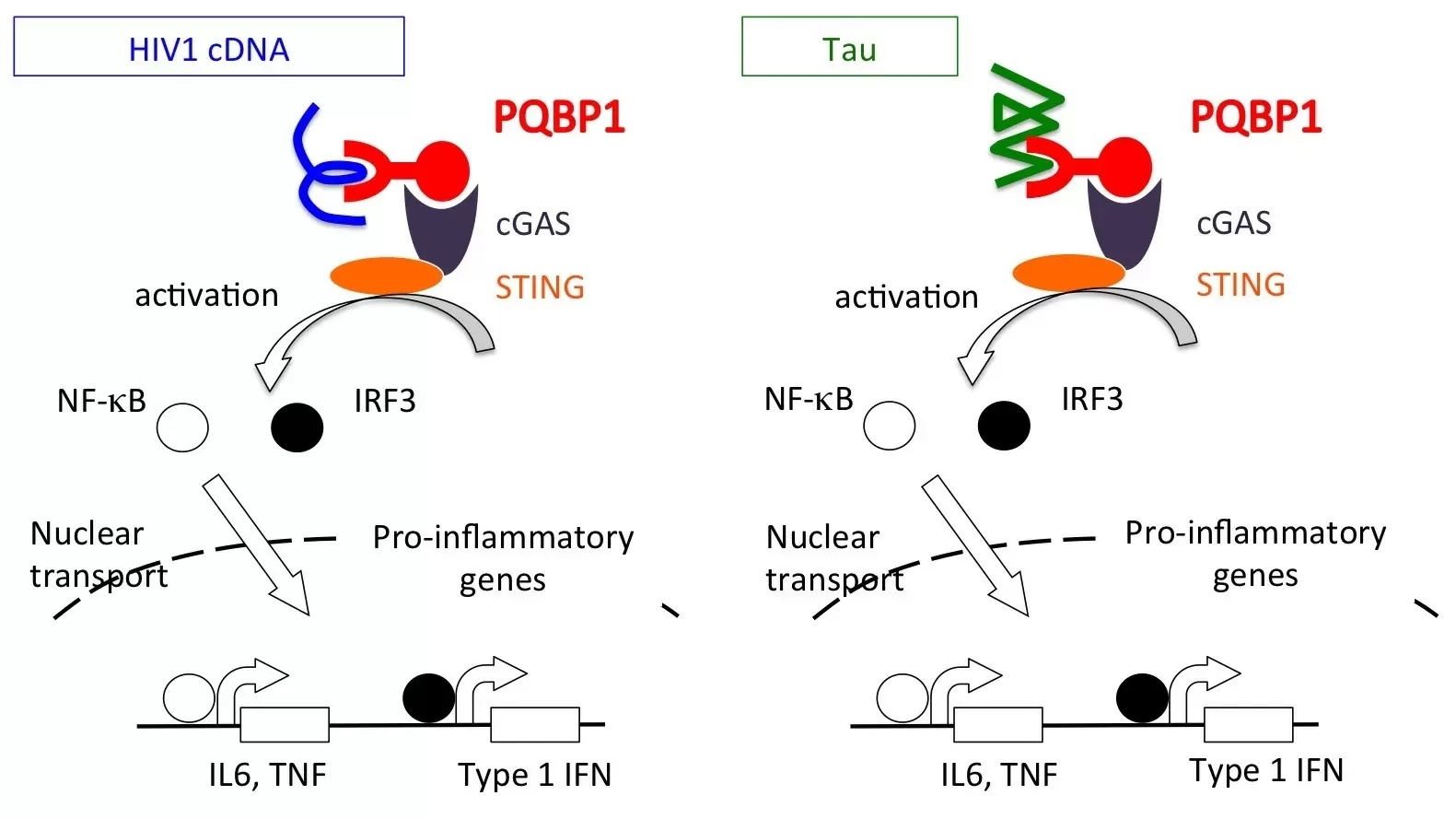
Figure 1: PQBP1, a new intracellular receptor for viral infection and neurodegeneration.
PQBP1 functions in brain microglia, which corresponds to dendritic cells or a part of macrophage regulating innate immun system, as an intracellular receptor for HIV cDNA and Tau proteins similarly.
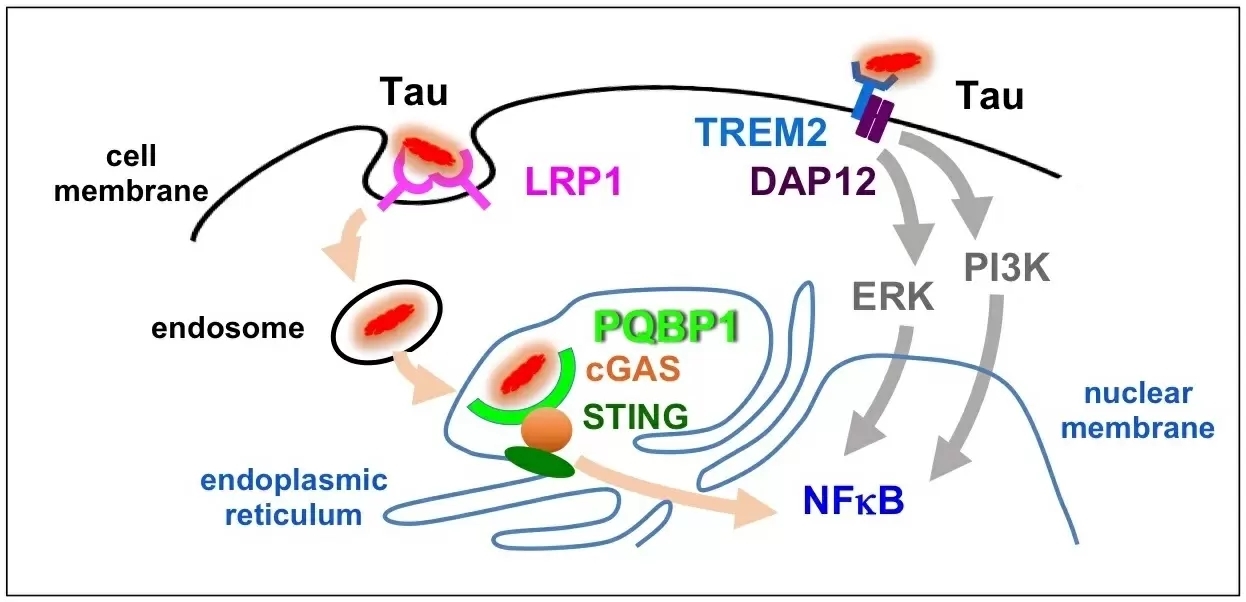
Figure 2: Two pathways independently activate microgrlia.
Tau protein is incorporated into microglia by LRP1 cell surface receptor and then binds to PQBP1 to activate cGAS-STING pathway and expression of pro-inflammatory genes. Meanwhile, Tau binding to TREM2 activates other signal pathway.
We further found that mutations in the PQBP1-binding regions of Tau reduced the inflammation in the brain in response to Tau injection. These findings indicate that PQBP1 may represent a potential target for the development of therapeutics for the treatment of Tau-mediated neurodegenerative diseases.
Publications
Tau activates microglia via the PQBP1-cGAS-STING pathway to promote brain inflammation.
Nat. Commun. 15 November 2021, 12 (1), 6565. doi: 10.1038/s41467-021-26851-2

