About PI
Takao Nakata
MD., Ph.D. Principal Investigator
Professor, Department of Cell Biology
Tokyo Medical and Dental University1-5-45
Yushima, Bunkyo-ku, Tokyo, Japan
113-8510
MD., Ph.D. Principal Investigator
Professor, Department of Cell Biology
Tokyo Medical and Dental University1-5-45
Yushima, Bunkyo-ku, Tokyo, Japan
113-8510
| Date of Birth | December 26, 1962 |
|---|---|
| 1987- | M.D., University of Tokyo, Medical School |
| 1988- | Assistant Professor, Department of Anatomy and Cell Biology, University of Tokyo, Faculty of Medicine |
| 1992- | Lecturer, Department of Anatomy and Cell Biology, University of Tokyo, Faculty of Medicine |
| 1994- | Associate Professor, Department of Anatomy and Cell Biology, University of Tokyo, Faculty of Medicine |
| 1994- | Ph.D., Department of Anatomy and Cell Biology, University of Tokyo, Faculty of Medicine |
| 2008- | Professor, Department of Cell Biology, Tokyo Medical and Dental University |
Major Findings of PI
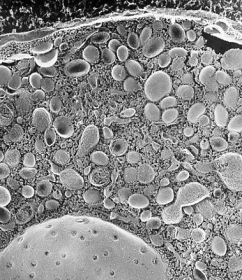
Professor Nakata started research as an electron microscopist in Hirokawa Laboratory, Department of Cell Biology, Medical School, University of Tokyo.
As an undergraduate student of medical school, he studied cytoskeleton of human platelets using quick-freeze, deep-etch, electron microscopy, and publised his first paper in JCB (T. Nakata, and N. Hirokawa. Cytoskeletal reorganization of human platelets after stimulation revealed by the quick-freeze deep-etch technique. Journal of Cell Biology 105(4): 1771-80. 1987.).
He studied the relationship between secretion and cytoskeletons using chromaffin cells, combining quick-freeze, deep-etch electron microscopy and cryo-immunoelectron microscopy. A micrograph of the chromaffin cells was selected for the cover picture in Journal of Neuroscience(T. Nakata, and N. Hirokawa. Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. Journal of Neuroscience 12(6): 2186-97. 1992.).
He also studied dynamin, which were thought to be a third microtubule motor protein, but concluded that dynamin was not a microtubule motor. He made a rigor-mutant of kinesin ATPase (T185N) to sutdy a role of kinesin in membrane traffick. During these studies, he was interested in membrane trafficking and noticed that characerization of cargo organelles were insufficient.
As an undergraduate student of medical school, he studied cytoskeleton of human platelets using quick-freeze, deep-etch, electron microscopy, and publised his first paper in JCB (T. Nakata, and N. Hirokawa. Cytoskeletal reorganization of human platelets after stimulation revealed by the quick-freeze deep-etch technique. Journal of Cell Biology 105(4): 1771-80. 1987.).
He studied the relationship between secretion and cytoskeletons using chromaffin cells, combining quick-freeze, deep-etch electron microscopy and cryo-immunoelectron microscopy. A micrograph of the chromaffin cells was selected for the cover picture in Journal of Neuroscience(T. Nakata, and N. Hirokawa. Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. Journal of Neuroscience 12(6): 2186-97. 1992.).
He also studied dynamin, which were thought to be a third microtubule motor protein, but concluded that dynamin was not a microtubule motor. He made a rigor-mutant of kinesin ATPase (T185N) to sutdy a role of kinesin in membrane traffick. During these studies, he was interested in membrane trafficking and noticed that characerization of cargo organelles were insufficient.
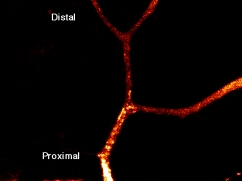
(1) Discovery of membrane organelles for constitutive secretory pathway
Secretion is classified into regulated (exocrine and endocrine cells) and constitutive (all cells) secretion. Organelles for the regulated secretion were well known as secretory vesicles, as they were accumulated in the cytoplasm. In contrast, organelles for constitutive secretion were not identified, although this pathway is important and transports albumin, collagen, immunogloblin, membrane receptors and channels. He identified organelles for constitutive secretory pathway, which were tubular globular organelles, heterogenous in shape, for the first time among the all type of cells(selected for cover in JCB (T. Nakata, S. Terada, and N. Hirokawa. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. Journal of Cell Biology 140(3): 659-74. 1998.) ).
He showed that synaptic vesicle proteins were also transported using the same pathway in the same paper.
Secretion is classified into regulated (exocrine and endocrine cells) and constitutive (all cells) secretion. Organelles for the regulated secretion were well known as secretory vesicles, as they were accumulated in the cytoplasm. In contrast, organelles for constitutive secretion were not identified, although this pathway is important and transports albumin, collagen, immunogloblin, membrane receptors and channels. He identified organelles for constitutive secretory pathway, which were tubular globular organelles, heterogenous in shape, for the first time among the all type of cells(selected for cover in JCB (T. Nakata, S. Terada, and N. Hirokawa. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. Journal of Cell Biology 140(3): 659-74. 1998.) ).
He showed that synaptic vesicle proteins were also transported using the same pathway in the same paper.
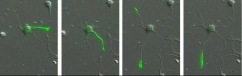
(2) Discovery of a novel feature of kinesin motor-head involved in the polarized vesicular transport in neurons
During the study of the mechanism for polarized transport in neurons, he noticed that kinesin motor domain accumulated at the tips of axons, but not dendrites, if its tail domain was cut. This finding suggests that microtubules are different between axons and dendrites, and kinesin motor head can recognize it, if inhibitory tail domain was deleted. This property of kinesin is now widely recognized as one of the earliest marker of polarization of hippocompal neurons.
(T. Nakata and N. Hirokawa. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. Journal of Cell Biology 162(6): 1045-55. 2003)
During the study of the mechanism for polarized transport in neurons, he noticed that kinesin motor domain accumulated at the tips of axons, but not dendrites, if its tail domain was cut. This finding suggests that microtubules are different between axons and dendrites, and kinesin motor head can recognize it, if inhibitory tail domain was deleted. This property of kinesin is now widely recognized as one of the earliest marker of polarization of hippocompal neurons.
(T. Nakata and N. Hirokawa. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. Journal of Cell Biology 162(6): 1045-55. 2003)
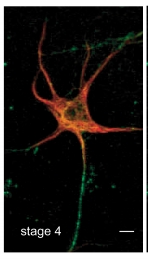
(3) Discovery of a novel feature of axonal microtubules in neurons
During the study of microtubules property of axonal microtubules, he found that axonal microtubules were heavily stained by GTP-tubulin specific antibody. High resolution light microscopy as well as in vitro assays supported that kinesin recognize a structural difference of axonal and dendrite microtubules due to the contents of GTP-tubulin.
(T. Nakata, S. Niwa, Y. Okada, F. Perez, and N. Hirokawa. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. The Journal of Cell Biology 194:245-255. 2011.)
細胞生物学分野日本語ページへのリンクはこちら
During the study of microtubules property of axonal microtubules, he found that axonal microtubules were heavily stained by GTP-tubulin specific antibody. High resolution light microscopy as well as in vitro assays supported that kinesin recognize a structural difference of axonal and dendrite microtubules due to the contents of GTP-tubulin.
(T. Nakata, S. Niwa, Y. Okada, F. Perez, and N. Hirokawa. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. The Journal of Cell Biology 194:245-255. 2011.)
細胞生物学分野日本語ページへのリンクはこちら

