A02-1

Investigation of proteomics
Research abstract
We have originally developed a new analyzing system of proteome generated by liquid chromatography and mass spectrometry (LCMS), named 2DICAL (2-Dimensional Image Converted Analysis of LCMS). Using the system, we have archived important success in the field of medicine and biology. 2DICAL analyzes the data of mass-to-charge ratio (m/z), peak intensity, retention time (RT), and each sample generated by liquid chromatography and mass spectrometry as the elemental data. It deployed various two-dimensional images with different combinations of axes using these four elements. From the m/z-RT image, peaks derived from the same peptide in the direction of acquiring time were integrated. By adding algorisms to ensure reproducibility of m/z and RT, the same peak was compared precisely across different samples, and a statistical comparison of identical peaks in different samples led to the discovery of specific differentially expressed peptide peaks.
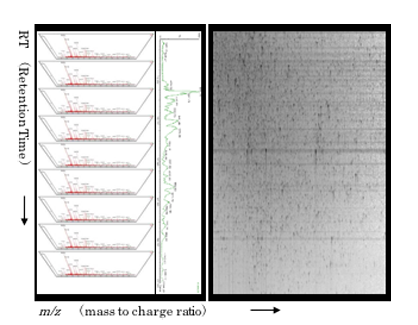
Basic image of 2DICAL
Combining each spectrum generated on retention time (RT), the intensity is plotted in the gray scale mode on the plane with the axes of m/z (mass to charge ratio) and RT.
Original papers
- Kamita, M., Mori, T., Sakai, Y., Ito, S., Gomi, M., Miyamoto, Y., Harada, A., Niida, S., Yamada, T., Watanabe, K., and Ono, M. (2015) Proteomic analysis of ligamentum flavum from patients with lumbar spinal stenosis. Proteomics 15, 1622-1630
- Kagami, Y., Nihira, K., Wada, S., Ono, M., Honda, M., and Yoshida, K. (2014) Mps1 phosphorylation of condensin II controls chromosome condensation at the onset of mitosis. J Cell Biology 205, 781-790
- Ono, M., Matsubara, J., Honda, K., Sakuma, T., Hashiguchi, T., Nose, H., Nakamori, S., Okusaka, T., Kosuge, T., Sata, N., Nagai, H., Ioka, T., Tanaka, S., Tsuchida, A., Aoki, T., Shimahara, M., Yasunami, Y., Itoi, T., Moriyasu, F., Negishi, A., Kuwabara, H., Shoji, A., Hirohashi, S., and Yamada, T. (2009) Prolyl 4-hydroxylation of a-fibrinogen a novel protein modification revealed by plasma proteomics. Biol. Chem. 284, 29041-29049
- Matsubara, J., Ono, M., Negishi, A., Ueno, H., Okusaka, T., Furuse, J., Furuta, K., Sugiyama, E., Saito, Y., Kaniwa, N., Sawada, J., Honda, K., Sakuma, T., Chiba, T., Saijo, N., Hirohashi, S., and Yamada, T. (2009) Identification of a Predictive Biomarker for Hematologic Toxicities of Gemcitabine. J. Clin. Oncol. 27, 2261-2268
- Ono, M., Shitashige, M., Honda, K., Isobe, T., Kuwabara, H., Matsuzuki, H., Hirohashi, S., and Yamada, T. (2006) Label-free quantitative proteomics using large peptide data sets generated by nanoflow liquid chromatography and mass spectrometry. Mol. Cell. Proteomics 5, 1338-1347
Reviews
- Ono, M., Kamita, M., Murakoshi, Y., Matsubara, J., Honda, K., Miho, B., Sakuma, T., and Yamada, T. (2012) Biomarker Discovery of Pancreatic and Gastrointestinal Cancer by 2DICAL: 2-Dimensional Image-Converted Analysis of Liquid Chromatography and Mass Spectrometry. Int J Proteomics 2012, 897412
- Ono, M., and Hakomori, S. (2004) Glycosylation defining cancer cell motility and invasiveness. Glycoconj. J. 20, 71-78