A02-1

Clarification of IRE1 shuttle zone between endoplasmic reticulum and golgi in cancer cells
Research abstract
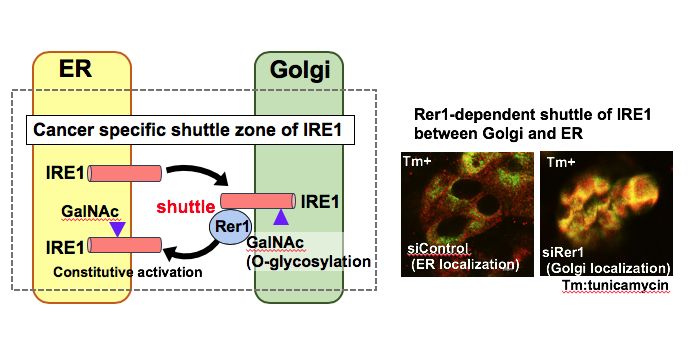
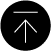
In solid tumors including breast cancers, endoplasmic reticulum (ER)-stress is constitutively activated due to infinite acceleration and tumor microenvironment such as hypoxia and glucose starvation. Although tumor cells may undergo necrosis or apoptosis if these conditions are severe, they have cellular survival strategies such as unfolded protein response (UPR). However, its molecular mechanism in carcinogenesis and progression is largely unknown. In our laboratory, we were attempting to elucidate the pathophysiological functions of one of GalNAc-T family, which is upregulated in a great majority of breast cancers and encodes a glycosyltransferase responsible for initiating mucin-type O-glycosylation in breast carcinogenesis and progression. Recently, we demonstrated that knockdown of one of GalNAc-T family expression by siRNA significantly suppressed breast cancer cell growth, and resulted in significant transcriptional down-regulation of the ER chaperons. More interestingly, we raised the possibility that O-glycosylation of IRE1 protein, an ER stress sensor, is required for its activation on UPR in breast cancer cells. In this study, we would like to clarify the molecular mechanism of IRE1 activation via its O-glycosylation though its visualization from ER to golgi apparatus, as a shuttle zone in cancer cells.
Original papers
- Kimura R, Yoshimaru T, Matsushita Y, Matsuo T, Ono M, Park JH, Sasa M, Miyoshi Y, Nakamura Y, *Katagiri T.
The GALNT6-LGALS3BP axis promotes breast cancer cell growth. Int J Oncol., in press. - Daizumoto, K., Yoshimaru, T., Matsushita, Y., Fukawa, T., Uehara, H., Ono, M., Komatsu, M., Kanayama, H., and Katagiri, T. (2018) A DDX31/mutant-p53/EGFR axis promotes multistep progression of muscle invasive bladder cancer. Cancer Res. 78,2233-2247
- Yoshimaru, T., Ono, M., Bando, Y., Chen, Y. A., Mizuguchi, K., Shima, H., Komatsu, M., Imoto, I., Izumi, K., Honda, J., Miyoshi, Y., Sasa, M., and Katagiri, T. (2017) A-kinase anchoring protein BIG3 coordinates oestrogen signaling in breast cancer cells. Nat Commun. 8,15427
- Kim, N. H., Yoshimaru, T., Chen, Y., Matsuo, T., Komatsu, M., Miyoshi, Y, Tanaka, , Sasa, M., Mizuguchi, K., and Katagiri, T. (2105) BIG3 inhibits the estrogen-dependent nuclear translocation of PHB2 via multiple Karyopherin-alpha proteins in breast cancer cells. PLoS One 10, e0127707.
- Yoshimaru, T., Komatsu, M., Matsuo, T., Chen, Y.A., Murakami Y, Mizuguchi K, Mizohata E, Inoue T, Akiyama M, Miyoshi Y, Sasa M, Nakamura Y., and Katagiri, T. (2013) Targeting BIG3-PHB2 interaction to overcome tamoxifen resistance in breast cancer cells. Nat Commun. 4, 2443.
- Park, J. H., Nishidate, T., Kijima, K., Ohashi, T., Takegawa, K., Fujikane, T., Hirata, K., Nakamura, Y., and Katagiri, T. (2010) Critical roles of MUC1 glycosylation by transactivated GALNT6 (UDP-N-acetyl-D- galactosamine: polypeptide N-acetylgalactosaminyl transferase-6) in mammary carcinogenesis. Cancer Res. 70, 2759-2769.