A02-2

研究概要
自ら開発した超解像ライブイメージング顕微鏡SCLIMを用いて、出芽酵母の小胞体の積荷蛋白質の輸送ゾーンに関する研究を行う。これまでに、積荷がCOPII被覆蛋白質集積部位(ER exit site; ERES)に一旦濃縮し、そこにゴルジ体のcis槽が接近、接触して積荷を受け取り(hug and kiss)再び離れる挙動を繰り返すことを明らかにしている(Nat Comm 2014)。このERESに集積する積荷の種類によって、異なる選別機構が働く可能性がある。実際、GPIアンカー蛋白質は、小胞体における脂質リモデリングによって特殊なマイクロドメインに濃縮され、非GPIアンカー型の積荷とは異なるERESから出芽することを支持するデータがすでに欧州のグループから発表されているが、イメージング技術の限界から、十分に説得力のある結論には至っていない。そこで本研究では、SCLIMを用い、GPIアンカー型および非GPIアンカー型の積荷を異なる蛍光蛋白質で標識し、酵母生細胞中のライブイメージングによって、両者が異なる部位に集積するかどうかをまず明らかにする。ここで異なる輸送ゾーンへの選別が明らかになれば、引き続きゴルジ体cis槽による積荷の受け取りを詳細に解析し、その後の分泌経路における両者の仕分けが継続するかどうかを明らかにする。この解析は、非常に正確な時間窓の中で積荷の挙動を追う必要があり、すでに確立したパルスチェイスイメージングの技法と、新たに開発した次世代型SCLIMを用いて初めて可能になるものである。積荷の種類によって異なる選別ゾーンが用いられ、複数の分泌輸送経路が存在することを示すことができれば、本領域の一つの柱である、機能ゾーンの並列によって複線の輸送経路を構成するという概念を実証でき、極めて画期的である。
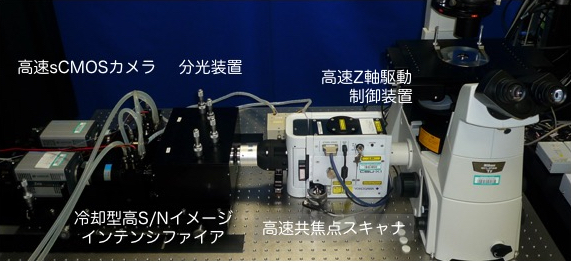
図:SCLIM顕微鏡
代表的な原著論文
該当研究者に下線(代表、分担)
- Shimizu, Y., Takagi, J., Ito, E., Ito, Y., Ebine, K., Komatsu, Y., Goto, Y., Sato, M., Toyooka, K., Ueda, T., Kurokawa, K., Uemura, T., and Nakano, A. (2021). Cargo sorting zones in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. Nat. Commun. 12:1901.
- Rizzo, R., Russo, D., Kurokawa, K., Sahu, P., Lombardi, B., Supino, D., Zhukovsky, M. A., Vocat, A., Pothukuchi, P., Kunnathully, V., Capolupo, L., Boncompain, G., Vitagliano, C., Marino, F. Z., Aquino, G., Montariello, D., Henklein, P., Mandrich, L., Botti, G., Clausen, H., Mandel, U., Yamaji, T., Hanada, K., Budillon, A., Perez, F., Parashuraman, S., Hannun, Y. A., Nakano, A., Corda, D., D’Angelo, G., and Luini, A. (2021). Golgi maturation-dependent glycoenzyme recycling controls glycosphingolipid biosynthesis and cell growth via GOLPH3. EMBO J. 40:e107238.
- Fujii, S., Kurokawa, K., Tago, T., Inaba, R., Takiguchi, A., Nakano, A., Satoh, T., and Satoh, A. (2020). Sec71 separates Golgi stacks in Drosophila S2 cells. J. Cell Sci. 133:jcs245571.
- Kanazawa, T., Morinaka, H., Ebine, K., Shimada, T. L., Ishida, S., Minamino, N., Yamaguchi, K., Shigenobu, S., Kohchi, T., Nakano, A., and Ueda, T. (2020). The liverwort oil body is formed by redirection of the secretory pathway. Nat. Commun. 11:6152.
- Rodriguez-Gallardo, S.†, Kurokawa, K.†, Sabido-Bozo, S., Cortes-Gomez, A., Ikeda, A., Zoni, V., Aguilera-Romero, A., Maria Perez-Linero, A., Lopez, S., Waga, M., Araki, M., Nakano, M., Riezman, H., Funato, K., Vanni, S., Nakano, A., and Muñiz, M. (2020). Ceramide chain length-dependent protein sorting into selective endoplasmic reticulum exit sites. Sci. Adv. 6:eaba8237. († equal contribution)
- Ikeda, A., Schlarmann, P., Kurokawa, K., Nakano, A., Riezman, H., and Funato, K. (2020). Tricalbins are required for nonvesicular ceramide transport at ER-Golgi contacts and modulate lipid droplet biogenesis. iScience 23:101603.
- Murakami-Sekimata, A., Sekimata, M., Sato, N., Hayasaka, Y., and Nakano, A. (2020). Deletion of pin4 suppresses the protein transport defects caused by sec12-4 mutation in Saccharomyces cerevisiae. Microbial Physiol. 30:25-35.
- Fujii, S., Kurokawa, K., Inaba, R., Hiramatsu, N., Tago, T., Nakamura, Y., Nakano, A., Satoh, T., and Satoh, A. K. (2020). Recycling endosomes attach to the trans-side of Golgi stacks in Drosophila and mammalian cells. J. Cell Sci. 133: jcs236935.
- Ishii, A., Kurokawa, K., Hotta, M., Yoshizaki, S., Kurita, M., Koyama, A., Nakano, A., and Kimura, Y. (2019). Role of Atg8 in the regulation of vacuolar membrane invagination. Sci. Rep. 9:14828.
- Shimada, T. L., Shimada, T., Okazaki, Y., Higashi, Y., Saito, K., Kuwata, K., Oyama, K., Kato, M., Ueda, H., Nakano, A., Ueda, T., Takano, Y., and Hara-Nishimura, I. (2019). HIGH STEROL ESTER 1 is a key factor in plant sterol homeostasis. Nat. Plants 5:1154-1166.
- Tojima, T., Suda, Y., Ishii, M., Kurokawa, K., and Nakano, A. (2019). Spatiotemporal dissection of the trans-Golgi network in budding yeast. J. Cell Sci. 132:jcs231159.
- Maeda, M., Kurokawa, K., Katada, T., Nakano, A., and Saito, K. (2019). COPII proteins exhibit distinct subdomains within each ER exit site for executing their functions. Sci. Rep. 9:7346.
- Shimada, T. L., Betsuyaku, S., Inada, N., Ebine, K., Fujimoto, M., Uemura, T., Takano, Y., Fukuda, H., Nakano, A., and Ueda, T. (2019). Enrichment of phosphatidylinositol 4,5-bisphosphate in the extra-invasive hyphal membrane promotes Colletotrichum infection of Arabidopsis thaliana. Plant Cell Physiol. 60:1514-1524.
- Abe, M., Kosaka, S., Shibuta, M., Nagata, K., Uemura, T., Nakano, A., and Kaya, H. (2019). Transient activity of the florigen complex during the floral transition in Arabidopsis thaliana. Development 146:dev171504.
- Kurokawa, K., Osakada, H., Kojidani, T., Waga, M., Suda, Y., Asakawa, H., Haraguchi, T., and Nakano, A. (2019). Visualization of secretory cargo transport within the Golgi apparatus in living yeast cells. J. Cell Biol. 218:1602-1618.
- Uemura, T., Nakano, R. T., Takagi, J., Wang, Y., Kramer, K., Finkemeier, I., Nakagami, H., Tsuda, K., Ueda, T., Schulze-Lefert, P., and Nakano, A. (2019). A Golgi-released subpopulation of the trans-Golgi network mediates protein secretion in Arabidopsis. Plant Physiol. 179:519-532.
-
Muro, K., Matsuura-Tokita, K., Tsukamoto, R., Kanaoka, M. M., Ebine, K., Higashiyama, T., Nakano, A., and Ueda, T. (2018) ANTH domain-containing proteins are required for the pollen tube plasma membrane integrity via recycling ANXUR kinases. Commun. Biol. 1:152.
-
Ishikawa, K., Tamura, K., Ueda, H., Ito, Y., Nakano, A., Hara-Nishimura, I., and Shimada, T. (2018) The synaptotagmin-associated ER-plasma membrane contact sites are distributed to immobile ER tubules. Plant Physiol. 178:641-653.
-
Haraguchi, T., Ito, K., Duan, Z., Sa, R., Takahashi, K., Shibuya, Y., Hagino, N., Miyatake, Y., Nakano, A., and Tominaga, M. (2018) Functional diversity of class XI myosins in Arabidopsis thaliana. Plant Cell Physiol. 59:2268-2277.
-
Ishii, M., Lupashin, V. V., and Nakano, A.(2018). Detailed analysis of the interaction of yeast COG complex. Cell Struct. Funct. 43:119-127.
-
Ito, E., Ebine, K., Choi, S.-W., Ichinose, S., Uemura, T.,Nakano, A., and Ueda, T. (2018). Integration of two RAB5 groups during endosomal transport in plants.eLife 7:e34064.
-
Minamino, N., Kanazawa, T., Era, A., Ebine, K., Nakano, A., and Ueda, T. (2018) RAB GTPases in the basal land plant Marchantia polymorpha. Plant Cell Physiol. 59:845-856.
-
Tanabashi, S., Shoda, K., Saito, C., Sakamoto, T., Kurata, T., Uemura, T., and Nakano, A. (2018) A missense mutation in the NSF gene causes abnormal Golgi morphology in Arabidopsis thaliana. Cell Struct. Funct. 43:41-51.
-
Takemoto, K., Ebine, K., Askani, J. C., Goh, T., Schumacher, K.,Nakano, A., and Ueda, T. (2018) Distinct sets of tethering complexes, SNARE complexes, and Rab GTPases mediate membrane fusion at the vacuole in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 115:E2457-E2466.
-
Suda, Y., Tachikawa, H., Inoue, I., Kurita, T., Saito, C., Kurokawa, K., Nakano, A., and Irie, K. (2018) Activation of Rab GTPase Sec4 by its GEF Sec2 is required for prospore membrane formation during sporulation in yeast Saccharomyces cerevisiae. FEMS Yeast Res. 18:fox095.
-
Sanchez-Rodriguez, C., Shi, Y., Kesten, C., Zhang, D., Sancho-Andrés, G., Ivakov, A., Lampugnani, E. R., Sklodowski, K., Fujimoto, M., Nakano, A., Bacic, A., Wallace, I. S., Ueda, T., van Damme, D., Zhou, Y., and Persson, S. (2018) The cellulose synthases are cargo of the TPLATE adaptor complex. Mol. Plant 11:346-349.
-
Ito, Y., Uemura, T., and Nakano, A. (2018) Golgi Entry Core Compartment functions as the COPII-independent scaffold for ER-Golgi transport in plant cells.J. Cell Sci. 131:jcs203893.
-
Yamagami, A., Saito, C., Sakuta, M., Shinozaki, M., Osada, H., Nakano, A., Asami, T., and Nakano, T. (2017) Brassinosteroids regulate vacuolar morphology in root meristem cells of Arabidopsisthaliana. Plant Signal. Behav. 13:e1417722.
-
Ito, Y., Toyooka, K., Fujimoto, M., Ueda, T., Uemura, T., and Nakano, A. (2017) The trans-Golgi network and the Golgi stacks behave independently during regeneration after Brefeldin A treatment in tobacco BY-2 cells. Plant Cell Physiol. 58:811-821.
-
Ishii, M., Suda, Y., Kurokawa, K., and Nakano, A.(2016) COPI is essential for Golgi cisternal maturation and dynamics. J. Cell Sci. 129:3251-3261.
-
Kurokawa, K., Suda Y., and Nakano, A. (2016) Sar1 localizes at the rims of COPII-coated membranes in vivo. J. Cell Sci. 129:3231-3237.
-
Kurokawa, K., Okamoto, M., and Nakano, A. (2014) Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat.Commun. 5:3653.
-
Uemura, T., Suda, Y., Ueda, T., and Nakano, A. (2014) Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 55:694-670.
-
Suda, Y., Kurokawa, K., Hirata, R., and Nakano, A.< (2013) Rab GAP cascade regulates dynamics of Ypt6 during the Golgi maturation. Proc.Natl. Acad. Sci. U. S. A. 110:18976-18981.
-
Ito, Y., Uemura, T., Shoda, K., Fujimoto, M., Ueda, T., Nakano, A. (2012) cis-Golgi proteins accumulate near the ER exit sites and act as the scaffold for Golgi regeneration after brefeldin A treatment in tobacco BY-2 cells. Mol. Biol. Cell 23:3203-3214.
-
Okamoto, M., Kurokawa, K., Matsuura-Tokita, K., Saito, C., Hirata, R., and Nakano, A. (2012) High-curvature domains of the endoplasmic reticulum (ER) are important for the organization of ER exit sites in Saccharomyces cerevisiae.J. Cell Sci. 125:3412-3420.
-
Uemura, T., Kim, H., Saito, C., Ebine, K., Ueda, T., Schulze-Lefert, P., and Nakano, A. (2012) Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc. Natl. Acad. Sci. U. S. A. 109:1784-1789.
総説
- Kurokawa, K. and Nakano, A. (2020). Live-cell imaging by super-resolution confocal live imaging microscopy (SCLIM): simultaneous three-color and four-dimensional live cell imaging with high space and time resolution. Bio-protocol 10:e3732.
- Nakano, A. and von Blume, J. (2019). Organelle zones. Mol. Biol. Cell 30:731.
- Kurokawa, K., and Nakano, A. (2019) The ER exit sites are specialized ER zones for the transport of cargo proteins from the ER to the Golgi apparatus. J Biochem. 165, 109–114.
- Suda, Y., Kurokawa, K., and Nakano, A.(2018) Regulation of ER-Golgi transport dynamics by GTPases in budding yeast. Frontiers Cell Dev. Biol. 5:122
-
Robinson, D. G., Brandizzi, F., Hawes, C., and Nakano, A. (2015) Vesicles versus tubes: is ER-Golgi transport in plants fundamentally different from other eukaryotes? Plant Physiol. 168, 393-406
-
Ito, Y., Uemura, T., and Nakano, A.(2014) Formation and maintenance of the plant Golgi apparatus. Intl. Rev. Cell Mol. Biol. 310, 221-287
-
Kurokawa, K., Ishii, M., Suda, Y., Ichihara, A., and Nakano, A.(2013) Live cell visualization of Golgi membrane dynamics by super-resolution confocal live imaging microscopy. Methods Cell Biol. 118, 235-242
-
Suda, Y. and Nakano, A. (2012) The yeast Golgi apparatus. Traffic 13, 505-510
-
Nakano, A., and Luini, A. (2010) Passage through the Golgi. Curr. Opin. Cell Biol. 22, 471-478
-
Glick, B. S., and Nakano, A. (2009) Membrane traffic within the Golgi apparatus. Annu. Rev. Cell Dev. Biol. 25:113-132