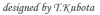
Top > Research Projects
![]()
I. Basophils play non-redundant roles in vivo, that are distinct from those played by mast cells
II. Pathogenesis of hyper-IgE syndrome
III. Regulation of B cell development
I. Basophils play non-redundant roles in vivo, that are distinct from those played by mast cells
Basophils are the least common leukocytes in the peripheral blood and account for only ~0.5% of them. Like mast cells, basophils express the high-affinity IgE receptor Fce RI on their cell surface, and they release chemical mediators such as histamine and leukotriene C4 upon stimulation. Therefore, basophils have often been neglected or considered minor and possibly redundant “circulating mast cells”, and analyzed as a surrogate of the less accessible tissue mast cells. The recent finding that basophils readily generate large quantities of Th2 cytokines such as IL-4 provided new insights into the possible role of basophils in allergic disorders and immunity to pathogens. However, in-depth studies on basophils, particularly their functions in vivo , have been hampered by the lack of appropriate animal models such as mutant animals deficient only in basophils. We have established allergen-specific IgE transgenic mice as an animal model of allergic disorders (Int. Immunol.,1999). Interestingly, a single subcutaneous injection of multivalent antigens elicited not only immediate- and late-phase ear swelling but also delayed-onset (starting on day 2 post-challenge) ear swelling with massive eosinophil infiltration ( J. Allergy Clin. Immunol., 2003). This was also true for normal mice that were passively sensitized with the IgE.. | 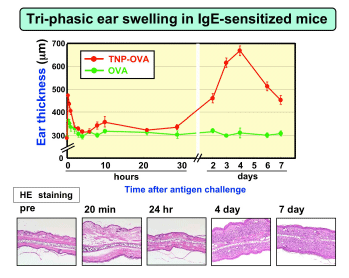 |
 |
Mast cells were essential for the immediate- and late-phase ear swelling but dispensable for the delayed one. T cells were also dispensable for the latter. The delayed-onset allergic inflammation was not developed in FceRI-deficient mice, but it was restored in these mice by transferring FceRI-expressing basophils from normal mice (Immunity, 2005). These findings indicate a novel mechanism of development of chronic allergic inflammation that is induced by basophils through the interaction of antigen, IgE, and FceRI. We have recently established a useful mAb, Ba103, that depletes only basophils when administered into mice (Blood, 2007; J. Immunol., 2007). The treatment of mice with Ba103 prior the Ag challenge almost completely abolished the development of the delayed-onset ear swelling. More importantly, the delayed-onset ear swelling was suppressed even when the mice were treated with Ba103 after ear swelling became evident. Even though basophils accounted for only 1-2% of the infiltrating cells in the skin lesions, the Ba103 treatment resulted in drastic reduction in numbers of not only basophils but also eosinophils and neutrophils infiltrating in the skin lesions. Thus, basophils play a pivotal role in the development of IgE-mediated chronic allergic inflammation, as an initiator rather than as an effector (Blood, 2007) . We are currently analyzing the molecular mechanism underlying the basophil-mediated chronic allergic inflammation by using a panel of engineered mice and the DNA microarray.
|
| References
1. Establishment of allergen-specific IgE transgenic mice as animal models of allergy Matsuoka, K., Taya, C., Kubo, S., Toyama-Sorimachi, N., Kitamura, F., Ra, C., Yonekawa, H. and Karasuyama, H.: Establishment of antigen-specific IgE transgenic mice to study pathological and immunobiological roles of IgE in vivo. Int. Immunol. 11: 987-994, 1999. (PubMed) 2. Chronic allergic inflammation mediated by basophils independently of T cells and mast cells Sato, E., Hirahara, K., Wada, Y., Yoshitomi, T., Azuma, T., Matsuoka, K., Kubo, Taya, C., Yonekawa, H., Karasuyama, H. and Shiraishi, A.: Chronic inflammation in skin can be induced in IgE transgenic mice by a single challenge of multivalent antigen. J. Allergy Clin. Immunol. 111: 143-148, 2003. (PubMed) Mukai, K., Matsuoka, K., Taya, C., Suzuki, H., Yokozeki, H., Nishioka, K., Hirokawa, K., Etori, M., Yamashita, M., Kubota, T., Minegishi, Y., Yonekawa, H., and Karasuyama, H.: Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23: 191-202, 2005. (PubMed) Obata, K., Mukai, K., Tsujimura, Y., Ishiwata, K., Kawano, Y., Minegishi, Y., Watanabe, N., and Karasuyama, H.: Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 110: 913-920, 2007. (PubMed) Kojima, T., Obata, K., Mukai, K., Sato, S., Takai , T. , Minegishi, Y., and Karasuyama, H .: Mast cells and basophils are selectively activated in vitro and in vivo through CD200R3 in an IgE-independent manner. J. Immunol. 179: 7093-7100, 2007 (PubMed). Tsujimura, Y., Obata, K., Mukai, K., Shindou, H., Yoshida, M., Nishikado, H., Kawano, Y., Minegishi, Y., Shimizu, T. and Karasuyama, H .: Basophils play a pivotal role in immunoglobulin G- but not immunoglobulin E-mediated systemic anaphylaxis. Immunity 2008 ( doi:10.1016/j.immuni.2008.02.008, published online on March 13, 2008 ) (PubMed). 3. Regulation of the high affinity receptor IgE (FceRI) expression on the cell surface Kubo, S., Matsuoka, K., Taya, C., Kitamura, F., Takai, T., Yonekawa, H. and Karasuyama, H.: Drastic up-regulation of high affinity IgE receptor on mast cells is induced by IgE binding through stabilization and accumulation of the receptor on the cell surface. J. Immunol. 167: 3427-34, 2001. (PubMed) Kubo, S., Nakayama, T., Matsuoka, K., Yonekawa, H., Karasuyama, H.: Long-term maintenance of IgE-mediated memory in mast cells in the absence of detectable serum IgE. J. Immunol. 170: 775-780, 2003. (PubMed) Kubota, T., Mukai, K., Minegishi, Y., and Karasuyama, H.: Different stabilities of the structurally-related receptors for IgE and IgG on the cell surface are determined by length of the stalk region in their α chains. J. Immunol. 176: 7008-7014, 2006. (PubMed) |

II. Pathogenesis of hyper-IgE syndrome
The hyper-IgE syndrome (HIES) is a compound primary immunodeficiency characterized by a highly elevated serum IgE, recurrent bacterial infections such as skin abscesses (so-called ‘cold abscesses') and cyst-forming pneumonia, severe chronic eczema indistinguishable from atopic dermatitis, and skeletal abnormalities. Most cases of HIES are sporadic, although some cases of familial HIES with autosomal dominant or recessive inheritance have been reported. In many cases (also called Job's syndrome), their clinical manifestations extend over multiple systems in the body, including the immune system, skeletal/dental system and soft tissue. In contrast, the abnormalities in familial autosomal recessive (AR)-HIES patients appear to be confined to the immune system. The gene(s) responsible for HIES and the molecular basis of the complex clinical manifestations have remained mysterious though the syndrome was described for the first time 40 years ago. (1) Identification of Tyk2 as a gene causative of AR-HIES We first investigated, from an immunological point of view, a patient who showed AR-HIES and susceptibility to infection with viruses and intracellular bacteria such as mycobacteria and salmonella. Peripheral blood cells from the patient displayed severe defects in the signaling pathways of multiple cytokines including type I IFN, IL-6, IL-10, IL-12 and IL-23. We identified a homozygous mutation of the Tyk2 gene in the patient, and his parents were both heterozygous for the same mutation (Immunity, 2006; Curr. Opin. Allergy Clin Immunol. 2007). Transduction of the intact gene rescued the patient's cells from the cytokine signaling defects, providing definitive evidence that the Tyk2 mutation was responsible for the HIES in the patient. The defects in multiple cytokine signals involved in innate and acquired immunity appeared to account for the complex clinical manifestations of the patient, including impaired Th1- and accelerated Th2-differentiation. The clinical phenotype of the human Tyk2 deficiency is much severer than that observed in the Tyk2 knock-out mice, indicating a species difference. Thus, Tyk2 plays indispensable roles in the human immune system. (2) Identification of STAT3 as a gene causative of classical HIES (Job's syndrome) The identification of a Tyk2 deficiency in AR-HIES prompted us to examine possible mutations in the Jak-STAT signaling axis in sporadic and classical HIES patients. We found that eight out of fifteen HIES patients had heterozygous STAT3 mutations, but their parents and siblings did not have the mutant STAT3 alleles, indicating these were de novo mutations (Nature, 2007). Five different mutations were found, all of which were located in the DNA-binding domain of STAT3. All five mutations were non-functional by themselves and showed dominant-negative effects when co-expressed with wild-type STAT3. Accordingly, the patients' peripheral blood cells showed defective responses to cytokines, including IL-6 and IL-10, the DNA-binding ability of STAT3 in these cells was greatly diminished. These results highlight the multiple roles played by STAT3 in humans, and underline the critical involvement of multiple cytokine pathways in the pathogenesis of HIES. The diagnosis of HIES early in life is often hampered by a paucity of specific clinical features. Our discovery of STAT3 as a major causative gene of this disease will facilitate earlier and definitive diagnosis, leading to the prevention of serious complications by prompting the start of prophylactic antibiotic treatment early in life.
References Minegishi, Y., Saito, M., Morio, T., Watanabe, K., Agematsu, K., Tsuchiya, S., Takada, H., Hara, T., Kawamura, N., Ariga, T., Kaneko, H., Kondo, N., Tsuge, T., Yachie, A., Sakiyama, Y., Iwata, T., Bessho, F., Ohishi, T., Joh, K., Imai, K., Kogawa, K., Shinohara, M., Fujieda, M., Wakiguchi, H., Pasic, S., Abinun, M., Ochs, H., D., Renner, E., D., Jansson, A., Belohradsky, B., H., Metin, A., Shimizu, N., Mizutani, S., Miyawaki, T., Nonoyama, S., and Karasuyama, H.: Human Tyk2 deficiency reveals requisite roles of Tyk2 in multiple cytokine signals involved in innate and acquired immunity.Immunity 25: 745-755, 2006 (PubMed) Minegishi, Y., Saito, M., Tsuchiya, S., Tsuge, I., Takada, H., Hara T., Kawamura, N., Ariga, T., Pasic, S., Stojkovic, O., Metin, A., and Karasuyama, H .: Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448: 1058-1062, 2007 (PubMed). Minegishi, Y. and Karasuyama, H.: Hyperimmunoglobulin E syndrome and tyrosine kinasae 2 deficiency. Curr. Opin. Allergy Clin Immunol . 7: 506?509, 2007 (PubMed). |

III. Regulation of B cell development
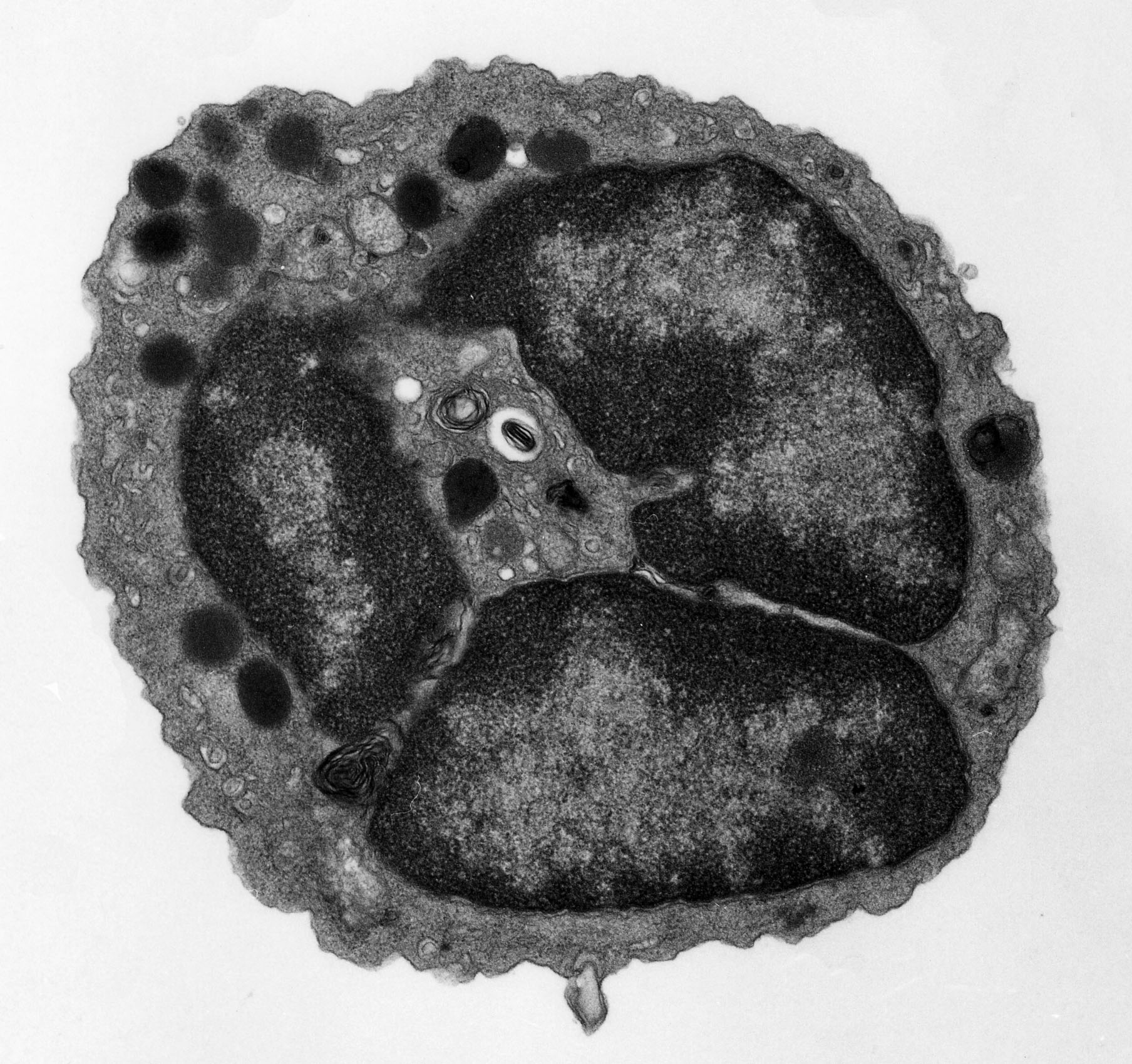
B cell development occurs in fetal liver during embryonic development and is subsequently maintained in the bone marrow of adult animals. During B cell development, V(D)J recombinations take place at IgH and IgL chain loci in an ordered fashion to create a diverse repertoire of B cell receptors. The preB cell receptor (preBCR), composed of mH chains, invariant VpreB/l5 surrogate light (SL) chains and signal transducing module Iga/Igb heterodimers, plays critical roles during the early stage of B cell development. Deficiency of preBCR formation or its signaling results in severe impairment of B cell differentiation at the transition from proB to preB cell stages in both humans and mice.
|  |
We have demonstrated that the Iga/Igb heterodimers are expressed on mH-negative proB cells and that the Igb cross-linking on proB cells in vivo induces early preB cell differentiation indistinguishable from that mediated by preBCR in normal B cell development
(Immunity, 1997). This provided a powerful tool to examine the preBCR signaling and its effects on proliferation and differentiation of preB cells in vitro and in vivo. By using this system, we showed that the preBCR signals regulated the accessibility at IgH and IgL chain loci (J. Exp. Med., 2000) and that Src family kinases played crucial roles in preBCR signaling (Nat. Immunol., 2003). We have recently established a novel system to distinguish two different populations among mH-expressing preB cells in fetal liver and adult bone marrow: one expressing mH chains capable of forming preBCR, and the other expressing mH chains incapable of forming preBCR. The former but not the latter population is positively selected for expansion and further differentiation in both fetal liver and adult bone marrow (Int. Immunol. 2005). We also found that preB cells expressing mH chains with particular VH sequences are positively selected and amplified through the preBCR checkpoint during their development in fetal liver, and their descendent B cells are maintained in the splenic marginal zone even in adult life, perhaps as innate-type B cells (Int. Immunol. 2005). We demonstrated that preBCR assesses the quality of IgH chains and tunes the preB cell repertoire by delivering differential signals (J. Immuno, 2006). The level of surface preBCR expression varies among preB cells, depending on the ability of their mH chains to pair with SL chains. The higher the level of preBCR expression by preB cells, the stronger their preBCR signaling, and the better they proliferate and differentiate. Thus, the extent of survival, proliferation and differentiation of individual preB cells is primarily determined by the SL-pairing ability of their mH chains. Furthermore, IgH chains with higher potential to assemble with IgL chains appear to be positively selected and amplified through the assessment of their ability to pair with SL chains at the preBCR checkpoint prior to the assembly into the BCR. These results indicate that the preBCR tunes the preB cell repertoire by driving preferential expansion and differentiation of cells with higher quality of mH chains. We are currently investigating the molecular mechanisms underlying the regulation of preBCR expression and signaling. The failure of this regulation appears to result in immunodeficiency, autoimmune diseases or malignant formation. | |
References
Nagata, K., Nakamura, T., Kitamura, F., Kuramochi, S., Taki, S., Campbell, K. S. and Karasuyama, H.: The Iga/Igb heterodimer on m-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity 7: 559-570, 1997. (PubMed) Maki, K., Nagata, K., Kitamura, F., Takemori, T. and Karasuyama, H.: Immunoglobulin b signaling regulates locus accessibility for ordered Ig gene rearrangements. J. Exp. Med. 191: 1333-1340, 2000. (PubMed) Saijo, K., Schmedt, C., Su, I-h., Karasuyama, H., Lowell, C.A., Reth, M., Adachi, T., Patke, A., Santana, A. and Tarakhovsky, A.: Essential role of Src-family protein tyrosine kinases in NF-kB activation during B cell development. Nature Immunol. 4: 274-279, 2003. (PubMed) Kawano, Y., Yoshikawa, S., Minegishi, Y., and Karasuyama, H.: Selection of stereotyped VH81X-mH chains via preB cell receptor early in ontogeny and their conservation in adults by marginal zone B cells.Int. Immunol. 17: 857-867, 2005 (PubMed) Kawano, Y., Yoshikawa, S., Minegishi, Y., and Karasuyama, H.: PreB cell receptor assesses the quality of IgH chains and tunes the preB cell repertoire by delivering differential signals. J. Immunol. 177: 2242-2249, 2006 (PubMed) |

