Dr. Tian from China stays in TMDU, Japan
A3 Foresight Program: Education Program for Young Researchers
Report of visit to Department of Molecular Oncology in TMDU (Feb. 15th to Fe. 22th, 2014)
Wei Tian (Department of Cancer Etiology, Peking University School of Oncology)
Host researcher
Prof. Yasuhito Yuasa, M.D Ph.D (Department of Molecular Oncology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University)
Summary
I visited Prof. Yuasa’s laboratory (Department of Molecular Oncology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo) in Japan to learn how to dissociate cells from gastric tumor xenografts from mouse, and culture these cells in vitro to examine whether these cells formed spheres, which have been considered to be a characteristic of cancer stem cells. I also learned how to analyze the cell surface antigen profiles of dissociated cells with FACS. In addition, I also practiced implanting gastric tumor tissue fragments into nude mouse and injecting the dissociated cells into NOD-Scid mouse.
I deeply thank Prof. Yasuhito Yuasa, Dr. Hiroshi Fukamachi and other members in Department of Molecular Oncology of TMDU for giving me a chance to visit and learn a lot in the wonderful lab. I’m thankful for all of members in the lab.
Contents
1. Dissociation of cells from gastric tumors
1) Cut off tumors from mouse and keep cancer tissues in Hanks’Balanced Salt Solution(HBSS) with 0.1% Bovine Serum Albumin(BSA) at 4 ℃ on ice.
2) Under a dissecting microscope, cut the tissues into small fragments with disposable scalpels. For tissues which are relatively hard, 0.8x0.8x0.8mm maybe good, and for soft ones, 1.5x1.5x1.5mm would be good, because we are using stainless steel needles for implantation with the inner diameter of 1.2mm.
3) Wash the tissues with disposable pipettes with RPMI1640 supplemented with 10% Horse Serum (HS) with antibiotics.
4) Keep the tissues in the media overnight at 4 ℃ in a refrigerator, to remove bacteria in the tumor tissue.
5) Wash the tissue fragments with CMF-PBS to remove serum, bacteria and dead cells.
6) Keep the fragments in 1 ml of CMF-PBS, and add equal volume of 2xTrypsin-EDTA with 2xDNase to treat the tissues.
7) Incubate the tissue fragments in Trypsin-EDTA with DNase for 20min at 37 ℃.
8) Dissociate free cells from the tissue fragments by pipetting with pipettes with appropriate tip sizes.
9) Remove dissociated cells, and add them into RPMI1640 with 10% HS to inactivate Trypsin-EDTA. Add new trypsin-EDTA solution to tissue fragments to treat the tissues for another 15 min at 37 ℃.
10) Dissociate free cells as shown in 8).
2. Culture of dissociated cells.
1) Collect the cell suspension, and remove large cell mass using strainer (mesh size=40 micrometer). Collect the single cells, and determine the cell number by using hemocytometers.
2) Centrifuge cell suspension at 1000rpm for 5 min, remove the supernatant and dilute cells to make 2x105 cells/ml with REBM medium with B27 supplement and 10 μM Rock inhibitor.
3) Coat the wells with matrigel solution on ice. Incubate the plates at 37℃ for 20 min.
4) Seed the cells at 2x105 cells/well in 12 well-plates, and culture them at 37℃, in 5% CO2 in air.
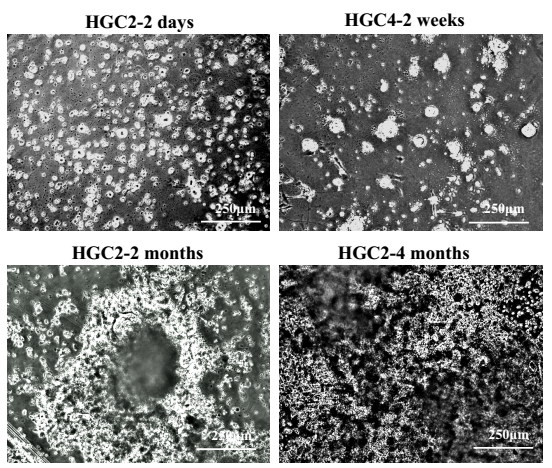
Figure1. Phase contrast micrographs of cells on the 4th day and 6th day in culture.
3. Analysis of the expression of CD49f by FACS.
1) Observe the cell spheres, which have been cultured for 2 weeks, 2 months, or 4 months, and took photos of them to record the cells growth. The results of HGC-2 cells, which have been cultured for 2 months or nearly 4 months, are showed in Figure 2.
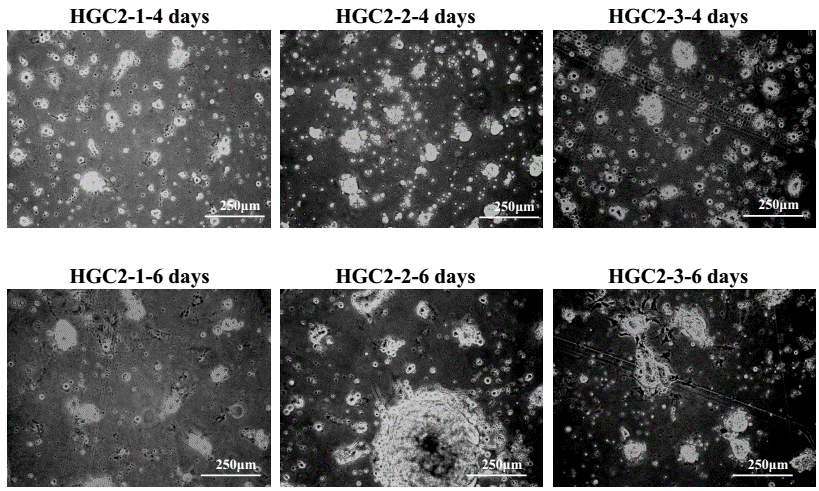
Figure 2. Phase contrast micrographs of cell spheres from different HGCs in primary culture.
2) Wash the cells on the plates with CMF-PBS.
3) Treat the cells with 1xTrypsin-EDTA at 37 ℃ for 1-1.5 hours. Collected these cells and inactivate the trypsin with RPMI1640 with 10% HS.
4) Centrifuge the cell suspension, remove the supernatant, and dilute the cells in 1ml of CMF-PBS and remove large cell mass using strainers (mesh size=40 micrometer). Collect the single cells, and determine the cell number by using hemocytometers.
5) Divide these cells into different groups (105 cells in one eppendorf tube at least). For analysis of CD49f, remove CMF-PBS, and add 10μl FITC-anti-CD49f antibody to about 105 cells. For negative control, PBS was used, instead of the antibody. Incubate them at 4 ℃ for 30 min.
6) Wash the cells with 1ml CMF-PBS for 2 times.
7) Examine FITC signal by using a FACS machine. Results with HGC-2 cells, which have been cultured for 2 months or nearly 4 months, are showed in Figure 3.
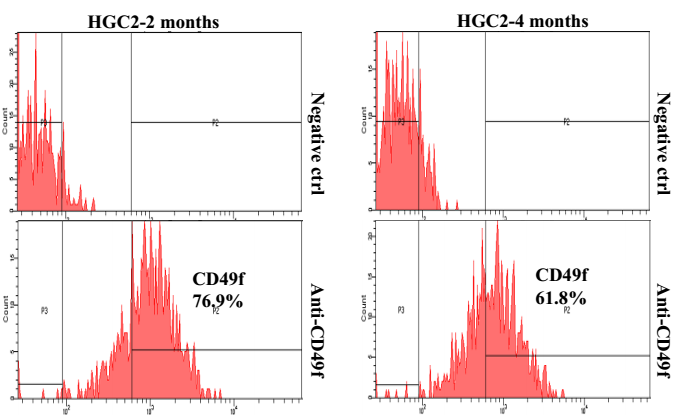
Figure 3. FACS analysis on the expression of CD49f on cultured cells.
4. Implantation of tissue fragments.
1) Cut off tumors from mouse and keep cancer tissues in HBSS with 0.1% BSA at 4 ℃ on ice.
2) Weigh the tissues and cut the tissues into small fragments with disposable scalpels. Keep some of them in formaldehyde solution for histochemical analyses.
3) Wash the tissues by pipetting with RPMI1640 supplemented with 10% HS with antibiotics.
4) Before implant these fragments into mouse, remove the serum by washing with HBSS. Put the small fragments into implantation needles.
5) When we inject the fragments into nude mouse, we firstly anesthesia them, then make a hole on the skin, and inject the fragments subcutaneously.
5. Discussion at TMDU.
During my stay in TMDU, I presented my data “Phosphatase of regenerating liver-3 directly interacts with integrin β1 and regulates its phosphorylation at tyrosine 783”and “IKBKE enhances cell-size and proliferation by phosphorylating p70S6K at Ser-427 in breast cancer” in lab meeting, and discussed with other laboratory members attending the meeting. They gave me some good suggestions about my work. It was very good for me to have the opportunity to discuss with the researchers.
Acknowledgments
I sincerely thank Prof. Yuasa for his invitation and kindly reception. I’m grateful to Dr. Fukamachi, not only for his patient instruction in experiments, but also for his thoughtful arrangement for my daily life. I thank Dr. Akiyama and Dr. Shimada, Sakamoto san, Nagasaki san and other members in the lab for their generous help and warm hospitality.




