Nucleic acid medicine
Research
Establishing new nucleic acid drug technologies
Nucleic acid drugs are short nucleic acids (oligonucleotides) with 6-30 bases as their basic framework, with a wide range of applications, such as inhibiting the expression of target RNA, RNA editing, and RNA-protein binding control, anticipated as the next generation of molecular targeted therapies. Nusinersen for spinal muscular atrophy improved motor function and life prognosis, becoming a significant turning point in the history of nucleic acid drug development.
While nucleic acid drugs have advanced in clinical applications, the limited efficacy in gene suppression with systemic administration has long been an issue. We developed Heteroduplex oligonucleotides (HDOs) as the "next generation nucleic acid drug" (Figure 1). HDOs are a new type of nucleic acid drug that enables it to introduce into all organs and cells, including the nervous system and achieves a 20-300-fold improvement in effectiveness compared to conventional nucleic acid drugs. HDOs have a different mechanism of action from ASO, a single-stranded DNA, and siRNA, a double-stranded RNA.
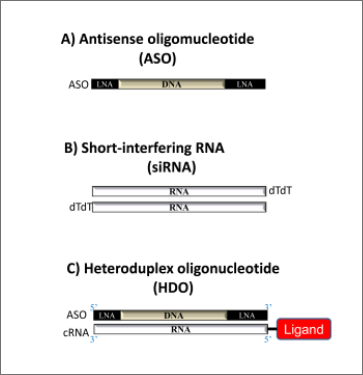
What is HDO?
Heteroduplex oligonucleotides (HDOs) are non-natural functional nucleic acids composed of a DNA backbone with antisense activity for binding to target RNAs and a complementary RNA strand. The central region of the double-stranded molecule consists of DNA-RNA heteroduplex, which is cleaved by the endonuclease RNaseH in the cell. As a result, the single-stranded DNA backbone binds to the target mRNA, and RNase H cleaves the target mRNA, leading to gene suppression (Figure 2A). This technology makes it possible to attach delivery ligands to the complementary RNA without affecting the binding affinity of the DNA backbone. For example, by binding proprietary vitamin E (VE) as a delivery ligand to HDOs, we achieved significantly higher target gene suppression compared to ASO or VE-binding siRNA (VE-siRNA) alone (Figure 2B).
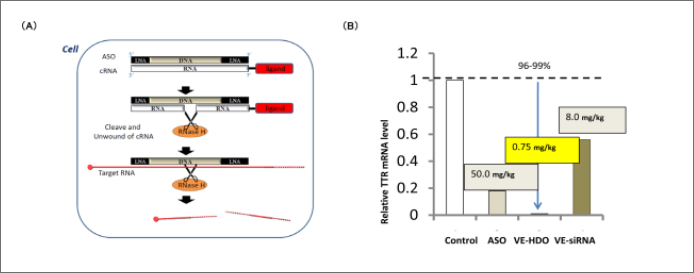
(A) HDO consists of a DNA main chain with antisense activity and a complementary RNA (cRNA). RNase H cleaves the cRNA at the center of the double-stranded region, leaving the single-stranded ASO. The ASO then binds to the target mRNA, suppressing gene expression.
(B) HDOs exhibit significantly higher efficacy in gene silencing than ASOs and siRNAs.
HDOs were initially limited to the liver with only intravenous injections. Our new molecular design technology can target gene regulation in major organs such as the heart, kidneys, subcutaneous fat, and skeletal muscle (Figure 3).
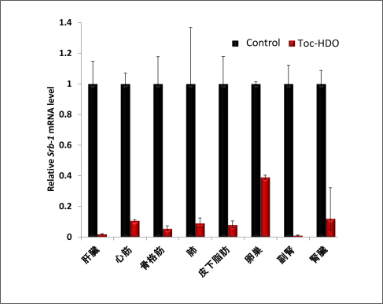
HDO intravenous administration allows endogenous target gene regulation in many abdominal organs other than the liver.
Blood-brain barrier penetrating heteroduplex nucleic acids
The biggest challenge in existing nucleic acid drugs is poor delivery to the central nervous system through systemic administration. Recently, we discovered a ligand molecule that can efficiently penetrate the blood-brain barrier (BBB) and successfully achieved significant inhibition of endogenous genes in the central nervous system, including the cerebrum, cerebellum, and spinal cord, which accounted for 70-90% of the targeted genes, by intravenously administering HDO bound to a ligand molecule. The administered HDOs were widely delivered to most neurons and glial cells in the cerebral cortex and white matter after passing through the BBB, and significant gene inhibition was achieved (Figure 4).
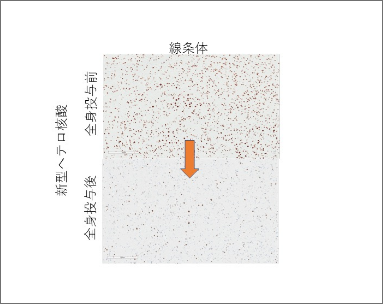
The novel HDO can suppress target genes in the striatum by passing through the blood-brain barrier after intravenous administration.
This BBB-penetrating HDO has been licensed to leading pharmaceutical companies in Japan and the United States as a new breakthrough technology. Based on these new foundational technologies, we have launched the AMED Advanced Bio-Medicines Developmental Platform "Molecular Targeted Therapy and Brain Imaging through the Development of Next-Generation BBB-Penetrating Heteroduplex Oligonucleotides" and have received numerous large public research grants. We have also collaborated with multiple major pharmaceutical companies in Japan and the United States and have filed more than 30 patents related to nucleic acid medicines.
The TIDE Center is collaborating with researchers in chemistry, genetic engineering, pharmacy, and molecular biology from around the world to advance drug discovery research for rare diseases using HDO technology as a standard for targeted therapy. We aim to create cutting-edge therapeutic technologies for rare diseases that currently have no effective treatment.
 JAPANESE
JAPANESE