Research
Research for the regulation of the blood-brain barrier function
The blood-brain barrier (BBB) is recognized as one of the crucial pathogenic sites in many neurological diseases such as multiple sclerosis, Alzheimer’s disease, and brain ischemia. The importance of regulating BBB function has been increasingly recognized in recent years. A platform technology that enables the regulation of BBB function has been developed as an innovative molecular targeted therapy for these diseases (Kuwahara H, et al. Ther Deliv 3: 417-420, 2012). Also, we reported that glucose transporter-1 expressed at remarkably high level at the BBB has a great influence on the pathogenesis of Alzheimer’s disease (Winkler EA, Nishida Y, Sagare AP, et al. Nat Neurosci 18: 521-30, 2015).
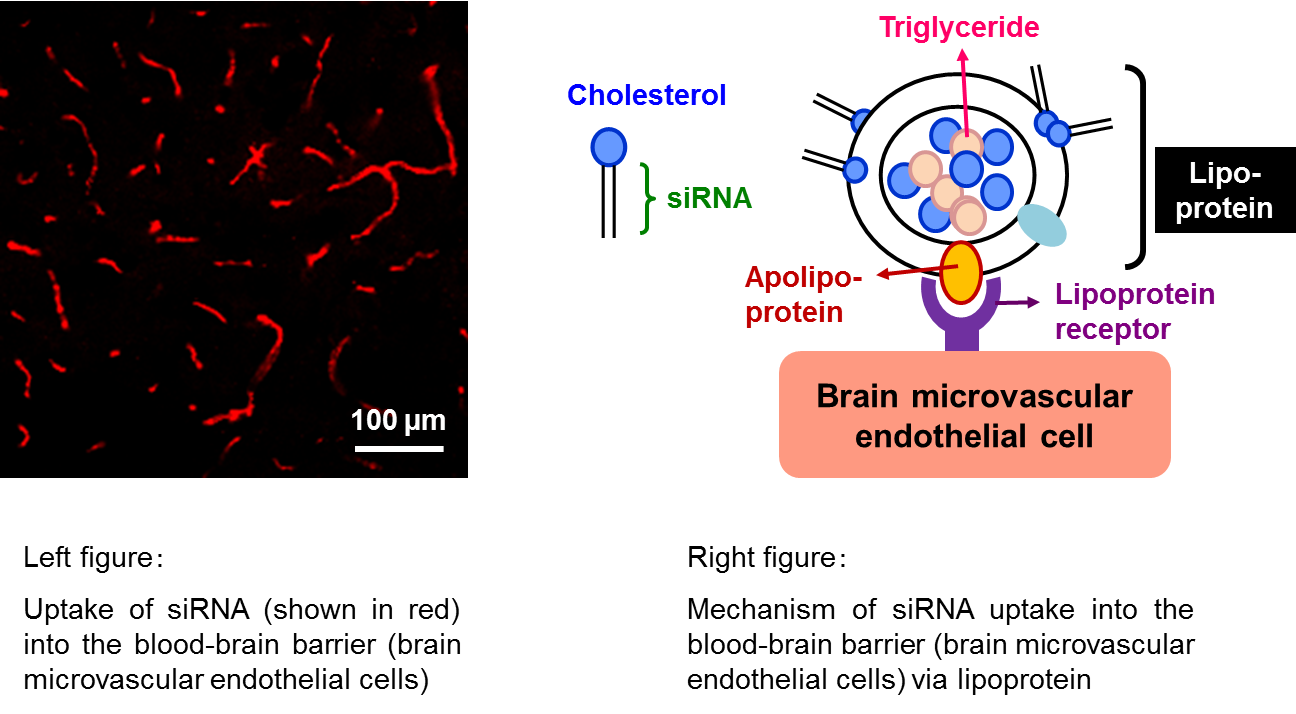
We have been leading the proof-of-concept research, which showed the short-interfering RNA (siRNA) delivered into the mouse BBB by systemic administration to induce RNA interference. Now, RNA interference is known to be a powerful tool to introduce gene silencing and expected to be developed as a novel technique for the future molecular targeted therapy. However, an efficient and safe delivery system in vivo is yet to be established for the clinical application. We first succeeded in an in vivo delivery of siRNA into the BBB by hydrodynamic injection into mice, which was the pioneer work of gene silencing in the BBB (Hino T, et al. Biochem Biophys Res Commun 340: 263-7, 2006). The hydrodynamic injection, however, cannot be used clinically because of its rapid loading of an extremely large volume of solution and subsequent injuries in various organs and tissues. We next focused on the physiological transport pathway of cholesterol in the blood circulation and developed a cholesterol-bound siRNA which was delivered by endogenous lipoprotein, resulting in an efficient gene silencing in the BBB upon conventional intravenous injection into mice (See figure, Kuwahara H, et al. Mol Ther 19: 2213-21, 2011).
We are now intensively seeking for a better strategy of BBB regulation both in efficiency and in safety by utilizing our original “hetero-duplex oligonucleotide” and a novel delivery technique remarkably enhancing the brain tropism. Furthermore, we are planning to verify the therapeutic effect of our technology on BBB regulation in the animal models with various neurological diseases, and are finally aiming at clinical application for the patients suffering from intractable neurological diseases.