Research
Elucidation of pathophysiology and development of therapeutic drugs for Alzheimer's disease
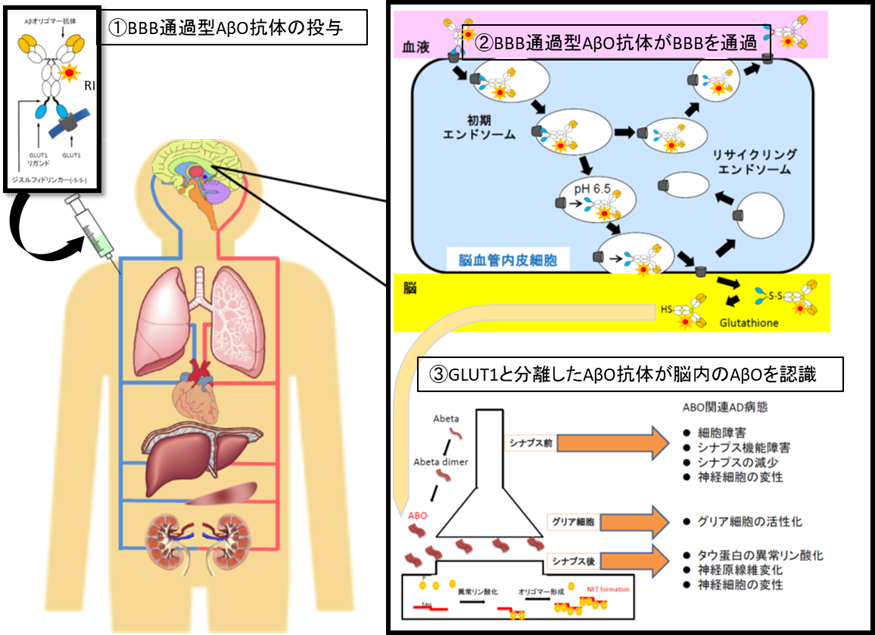
According to the announcement by the Ministry of Health, Labor and Welfare, in Japan, 15% of elderly people aged 65 years or older in 2012 are dementia, the elderly with dementia is about 4.62 million (1 in 7 people) Moreover, there are about 4 million ‘mild cognitive impairment’, which is the preliminary stage of dementia, and about half of them are considered to be Alzheimer's disease (AD) in the near future. Since the incidence rate of Alzheimer's disease rapidly increases when the elder people become over 65 years of age, it is obvious that a development of effective treatment for dementia is urgently needed. AD has a pathological feature of senile plaque in which amyloid β protein (Aβ) is aggregated and deposited, neurofibrillary tangles composed of abnormal tau protein in neurons, and degeneration of neural cells. In recent years, it was revealed that amyloid β oligomer (AβO) which is a low molecular weight polymer of amyloid β protein (Aβ) has strong neurotoxicity and is strongly associated with pathophysiology in the early stage of the AD. Therefore, our group are generating a specific antibody against AβO, which is genetically engineered, and also using the BBB passage technology, and make the antibodies passing through into the brain. Then we are going to achieve the visualization of AβO in the patient brain before AD onset, and also the removal effect of AβO with the same antibodies, which is called as "Theranostics" for its dual effect of diagnosis and treatment by the same antibodies.
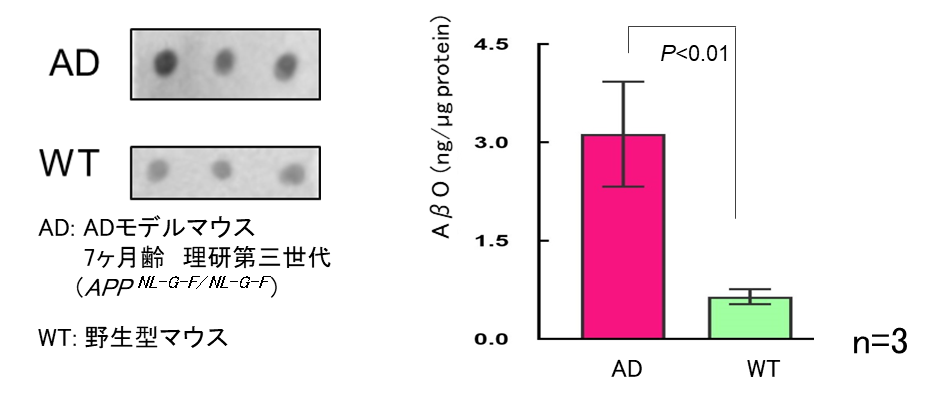
Quantitative evaluation of AβO, which has strong toxicity, is difficult using general biochemical analysis methods such as Western blotting for its instability. The evaluation of amyloid removal effect of antibody drug for AD have been conducted by counting senile plaques in the brain of model animals, instead. Recently we have developed a new measurement method of AβO in the brain, which make possible to measure successfully accurate soluble AβO in the brain of model mice.
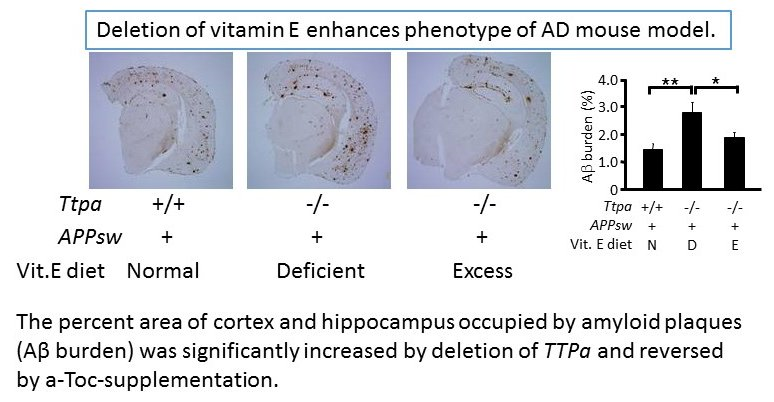
We also doing researches on the impact of oxidative stress and aging on AD. An early pathological event in AD is increased oxidative stress, which contributes to membrane damage, deterioration of synaptic plasticity and neuronal cell death, suggesting that oxidative stress promotes the initiation and/or progression of the disease. We generated α–tocopherol transfer protein (α-TTP) knockout (Ttpa-/-) mice which show marked lipid peroxidation in the brain because of a lack of α–tocopherol and are highly valuable as a model of chronic oxidative stress to the brain. By crossing AD transgenic model mice with Ttpa-/- mice, we obtained double mutant APPsw/Ttpa-/- mice, which showed increased Aβ deposits in the cerebrum and enhanced cognitive dysfunctions to be compared with APPsw mice (Nishida et al, BBRC 2006). The lipid peroxidation due to depletion of α-tocopherol impairs Aβ clearances from the brain and from the blood, possibly causing increased Aβ accumulation in Ttpa−/−APPsw mouse brain and plasma (Nishida et al, JBC 2009). Together with the fact that lipid peroxidation may be a major cause for aging of the brain, oxidative stress and aging are major contributors to AD pathogenesis. We further explored new genes and microRNA that would be involved in the pathogenesis of AD by mining micro array data using these model mice, and found several candidate genes including Pla2g3 which may play a role in cerebrum specific changes of AD pathology under chronic oxidative stress (Yui et al, PLoS One 2015). Understanding the molecular mechanism of how aging and oxidative stress accelerates AD pathophysiology will improve our understanding of fundamental cause of this disease and help us to develop novel treatment strategies for AD.